Increasing the breadth of influenza immunity through informed vaccine design
Kelsey Florek June 30, 2017
Slides available at:
www.k-florek.net/talks
how can vaccines be used to elicit protective immune responses that are not naturally occurring?
outline
- basics of influenza
- synopsis of immunology
- influenza adaptation to immune responses
- methods of protecting against influenza
- can vaccines stimulate a response that provides protection against different influenza subtypes?
- does ADCC play a role in human responses to seasonal influenza vaccination?
influenza virus
- negative sense RNA virus
- segmented genome
- characterized by glycoprotein spikes
- hemagglutinin (HA)
- neuraminidase (NA)
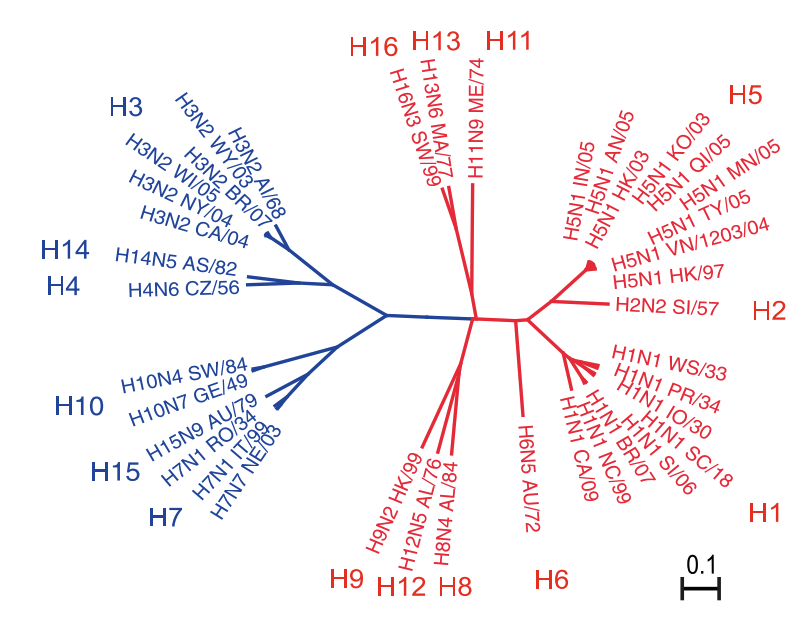
- subtype - H3N2 or H1N1
- strain - (H1N1) A/California/04/2009
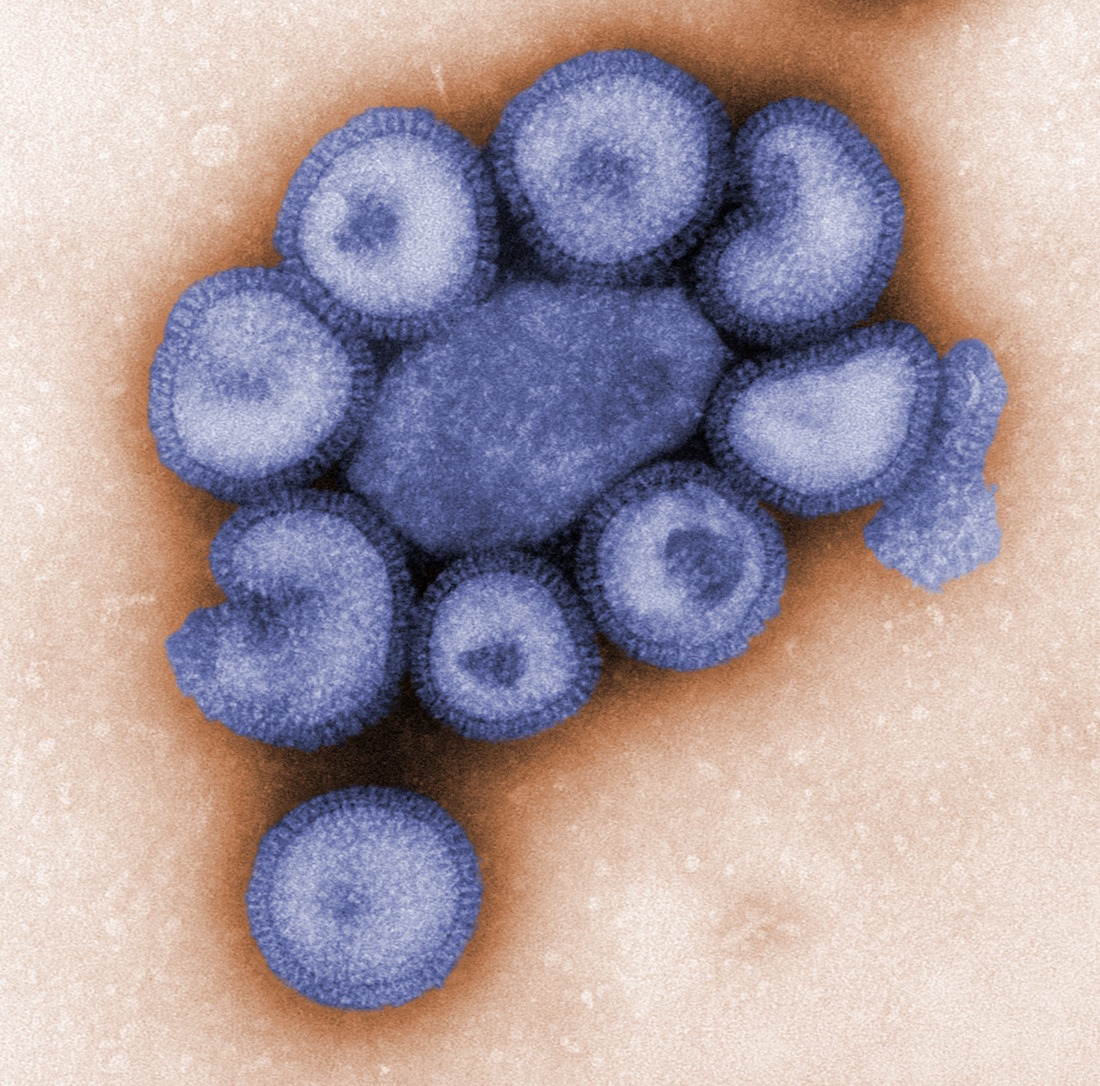
the origins of influenza today
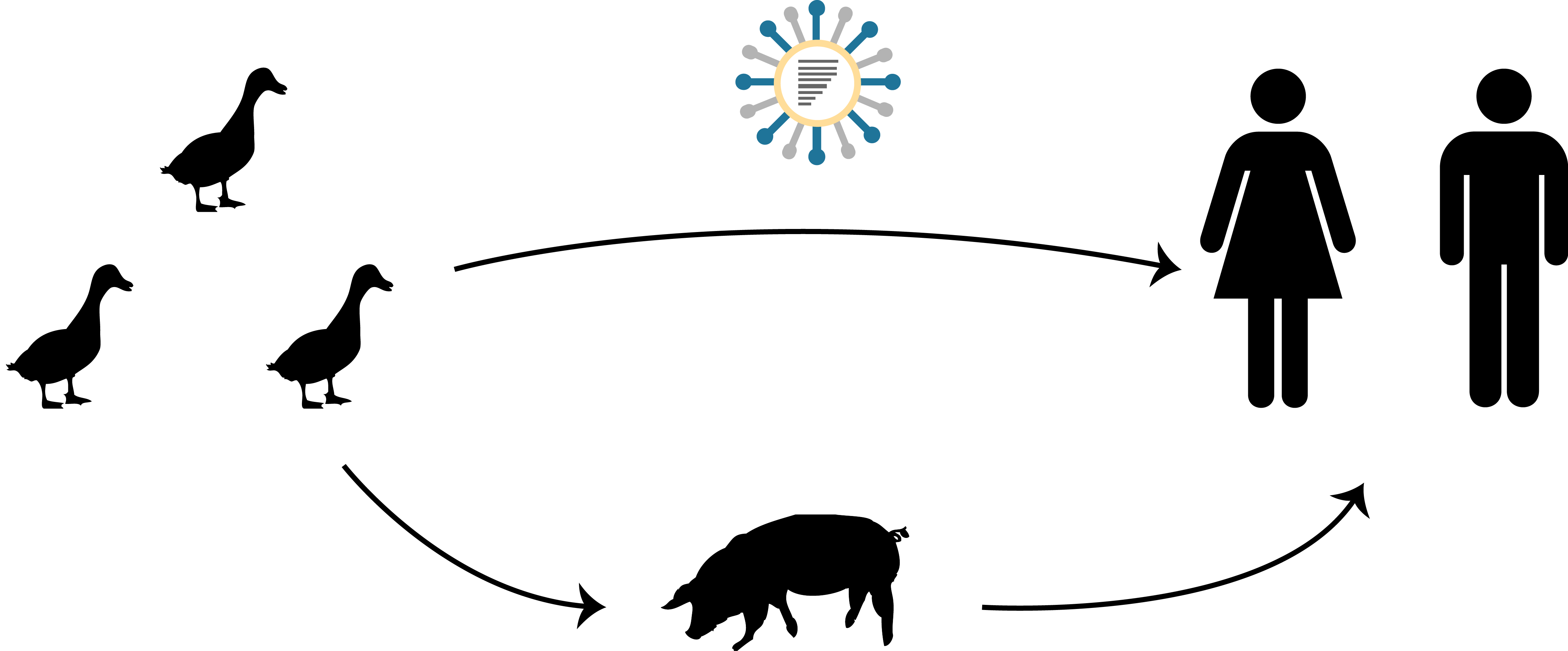
- pandemic - worldwide spread of a new influenza subtype
- seasonal - repeated spread influenza subtype associated with time of year
basis of immunity
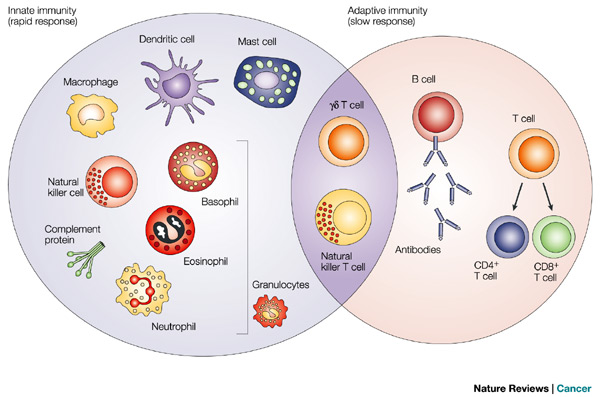
innate response - NK cells
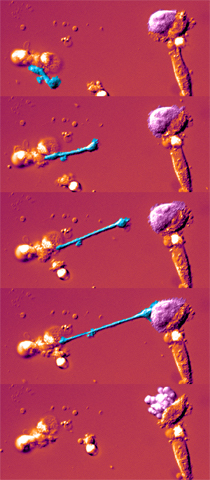
- role: kill infected and/or tumor cells - recognize altered self
- activation: balance of activating and inhibiting receptors
- function: small granules in the cytoplasm contain proteins (perforin and granzymes) that trigger apoptosis
adaptive response - T cells
T helper cell - CD4+
- role: assist other immune cells
- activation: T cell receptor engagement with antigen canonically presented from external sources
- function: release cytokines that regulate immune responses
cytotoxic T cell - CD8+
- role: kills infected and/or tumor cells
- activation: T cell receptor engagement with antigen canonically presented from internal sources
- function: release perforin and granzymes that trigger apoptosis
adaptive response - antibodies
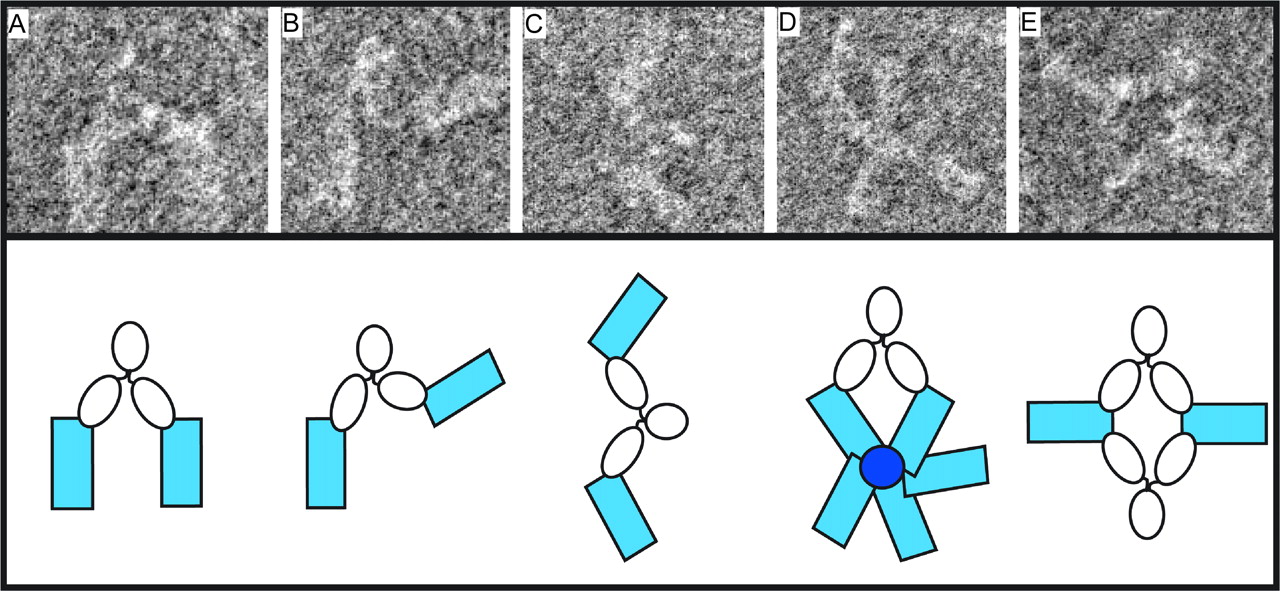
- role: recognize antigens
- function:
- neutralization - blocks functional parts of a bacteria or virus
- opsonization - tag foreign targets boosting the kinetics of phagocytosis
- complement activation - initialization of the complement membrane attack complex
- activate effector cells - engagement and activation of an immune effector cell
neutralizing antibody responses against influenza
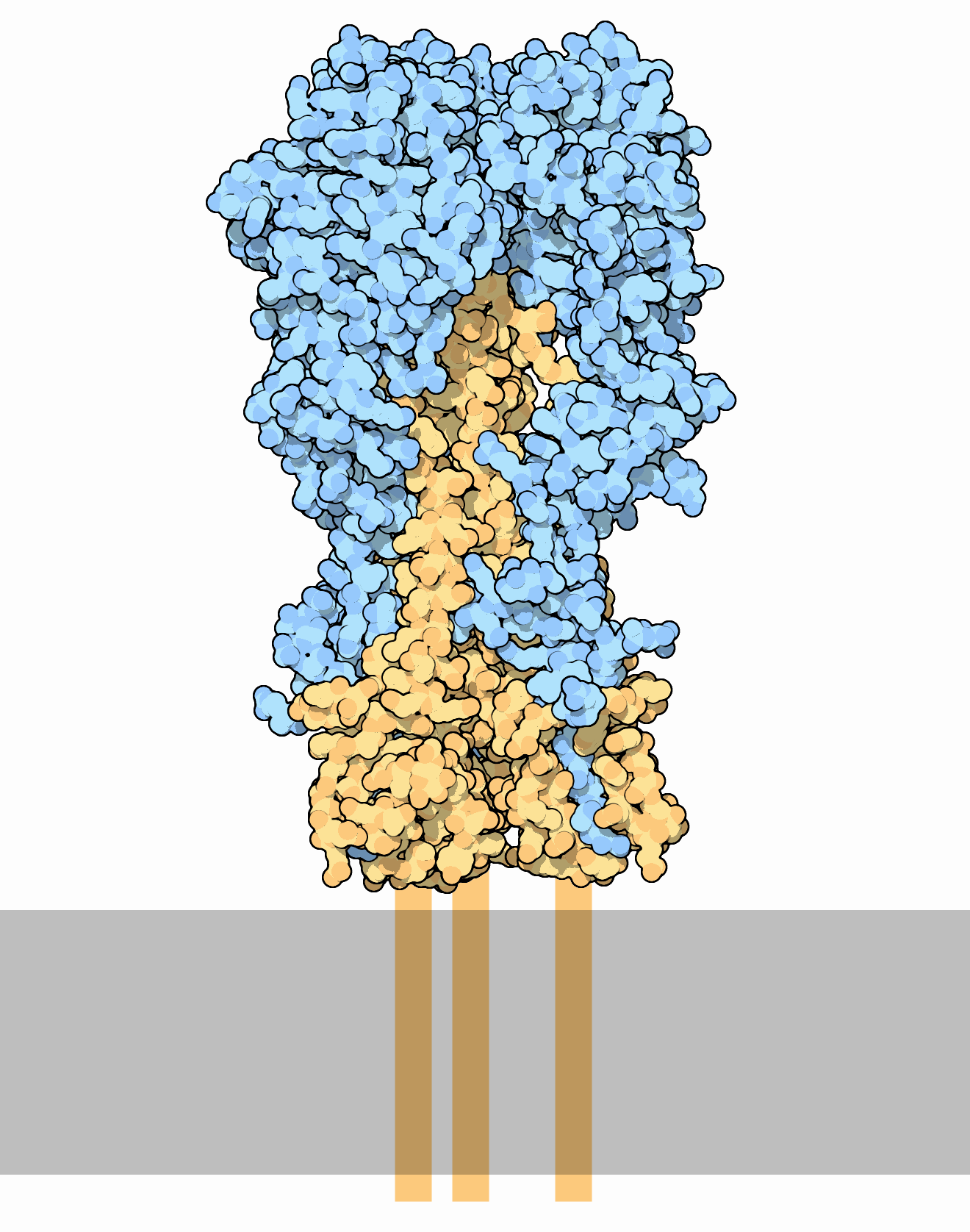
neutralizing antibody responses against influenza
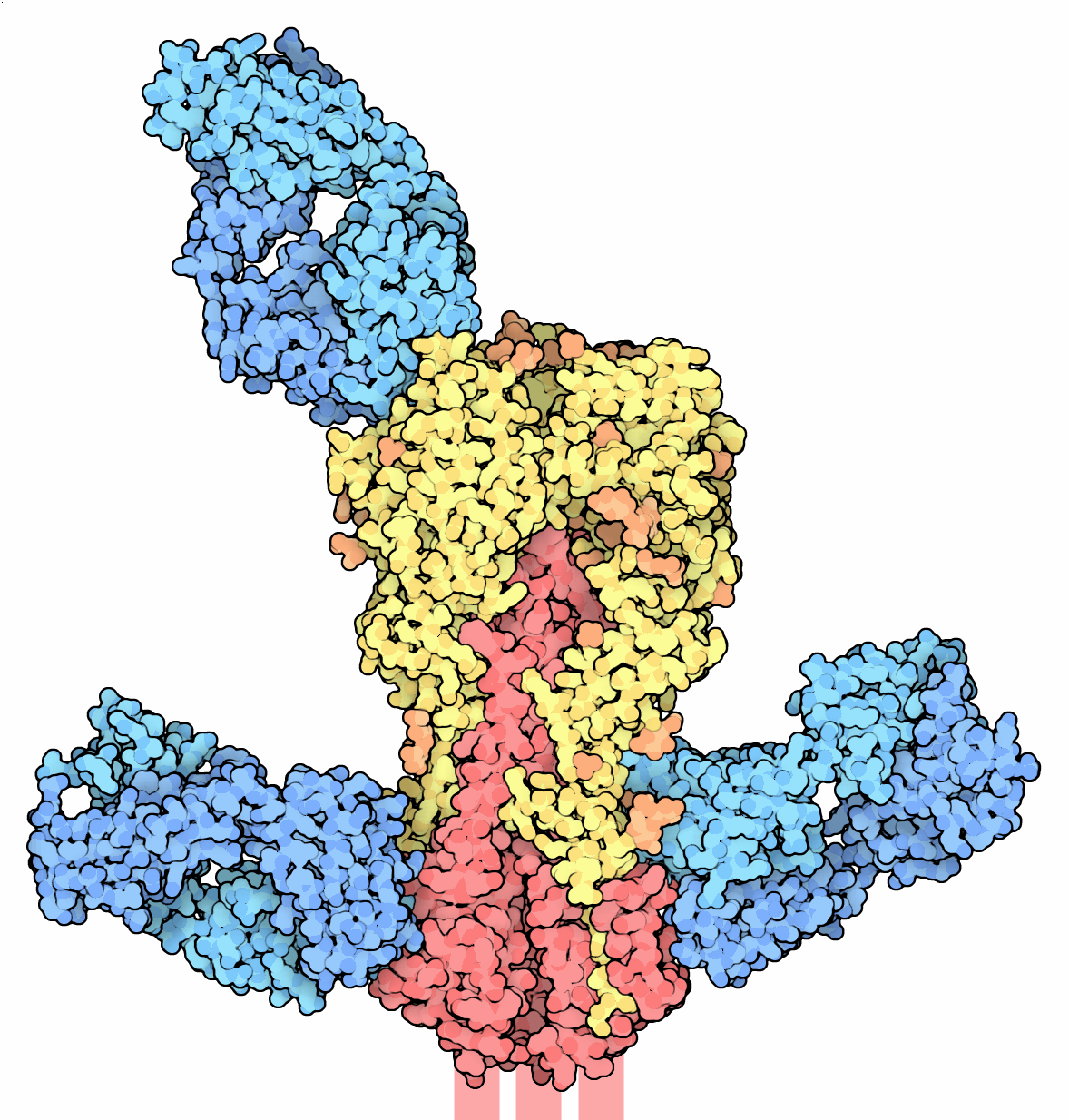
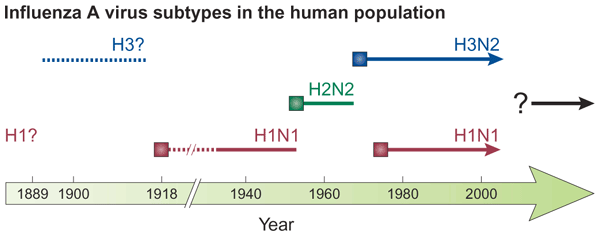
influenza adapts to immunity
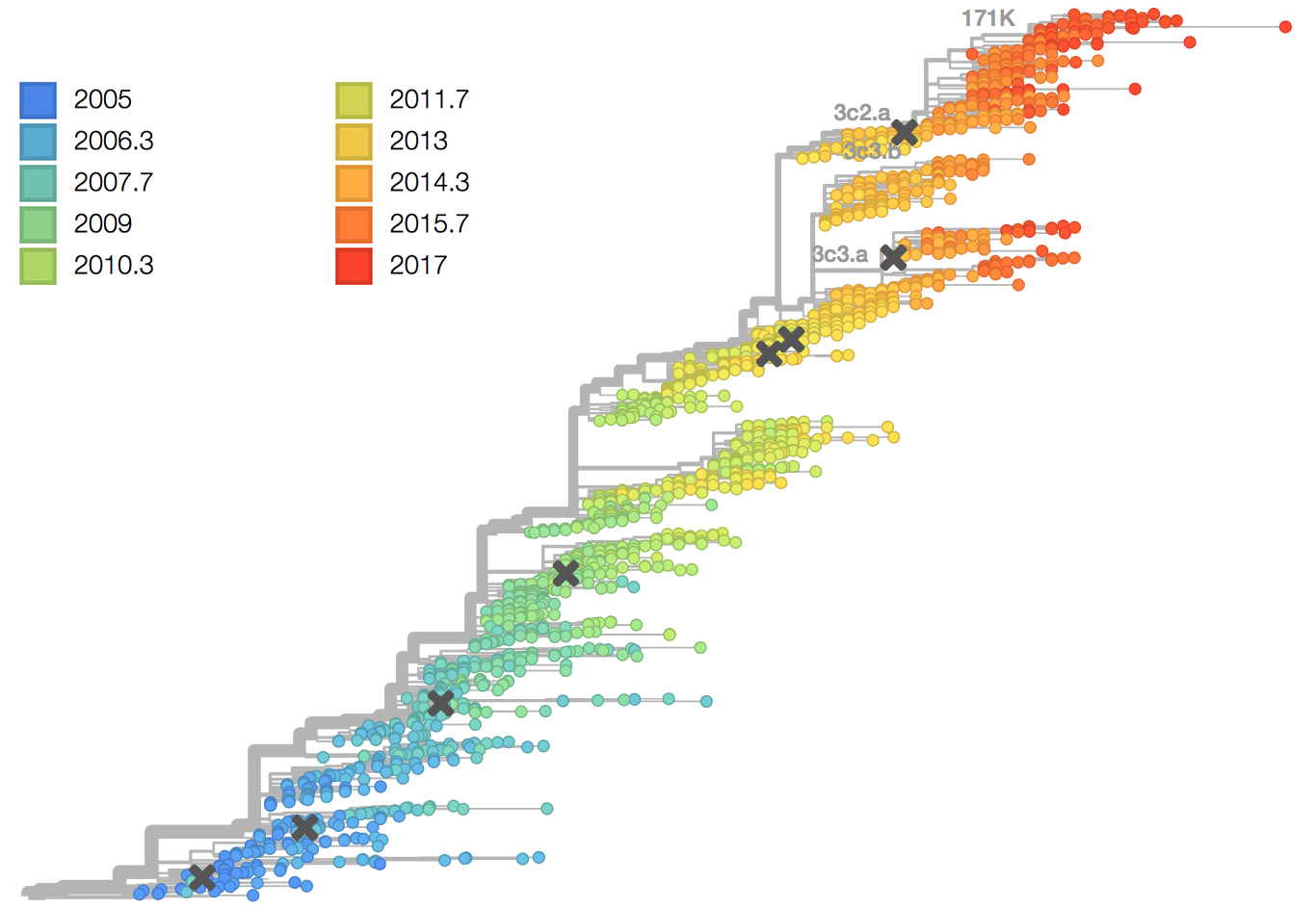
H3 evolution
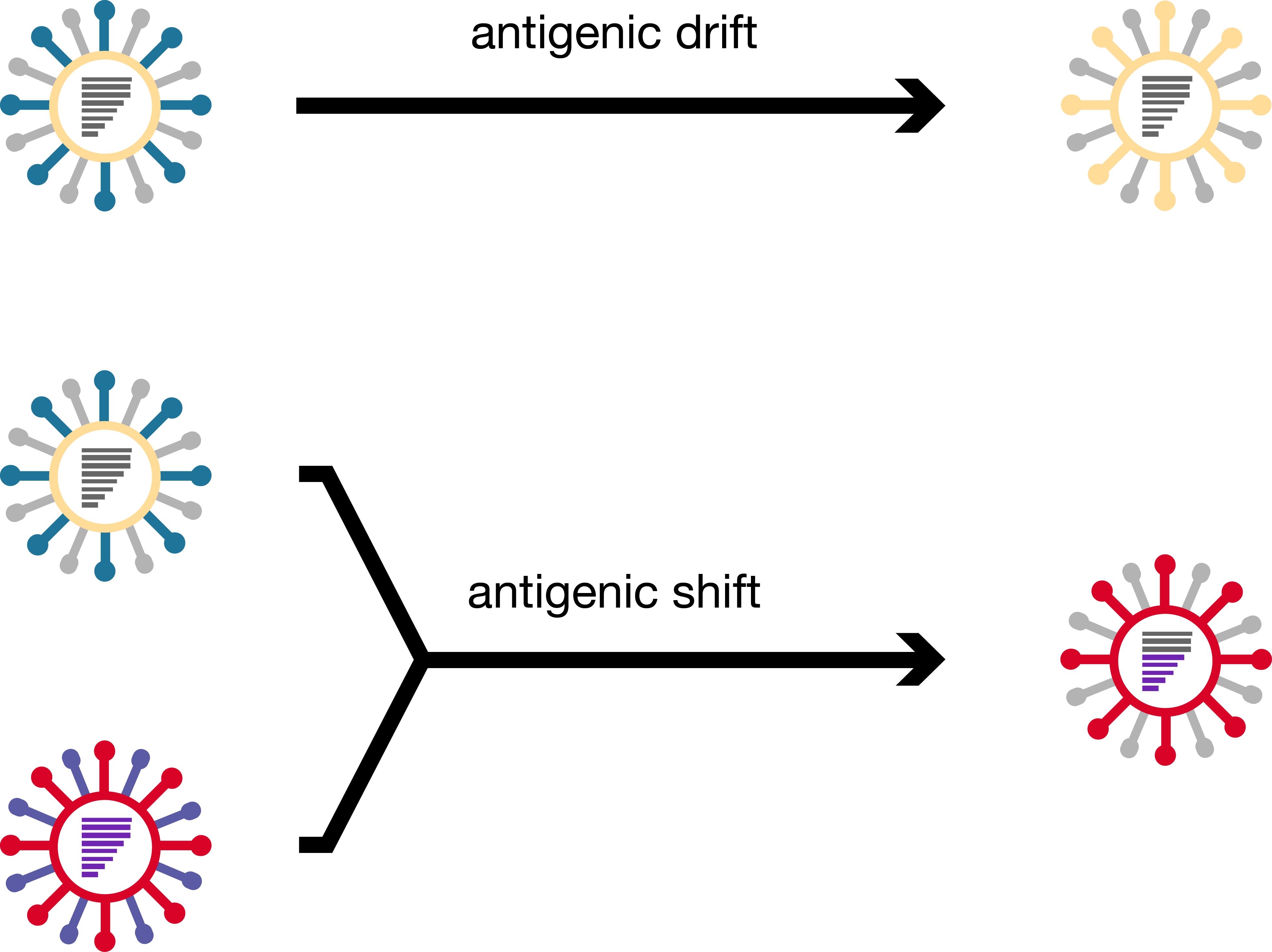
antigenic shift of HA
training the immune system to work against influenza - vaccines
- live attenuated influenza vaccine (LAIV) - virus particles are cold-adapted and replicate less efficiently in the human respiratory tract
- inactivated influenza vaccine (IIV) - virus particles are broken apart and only the proteins are used in the vaccine
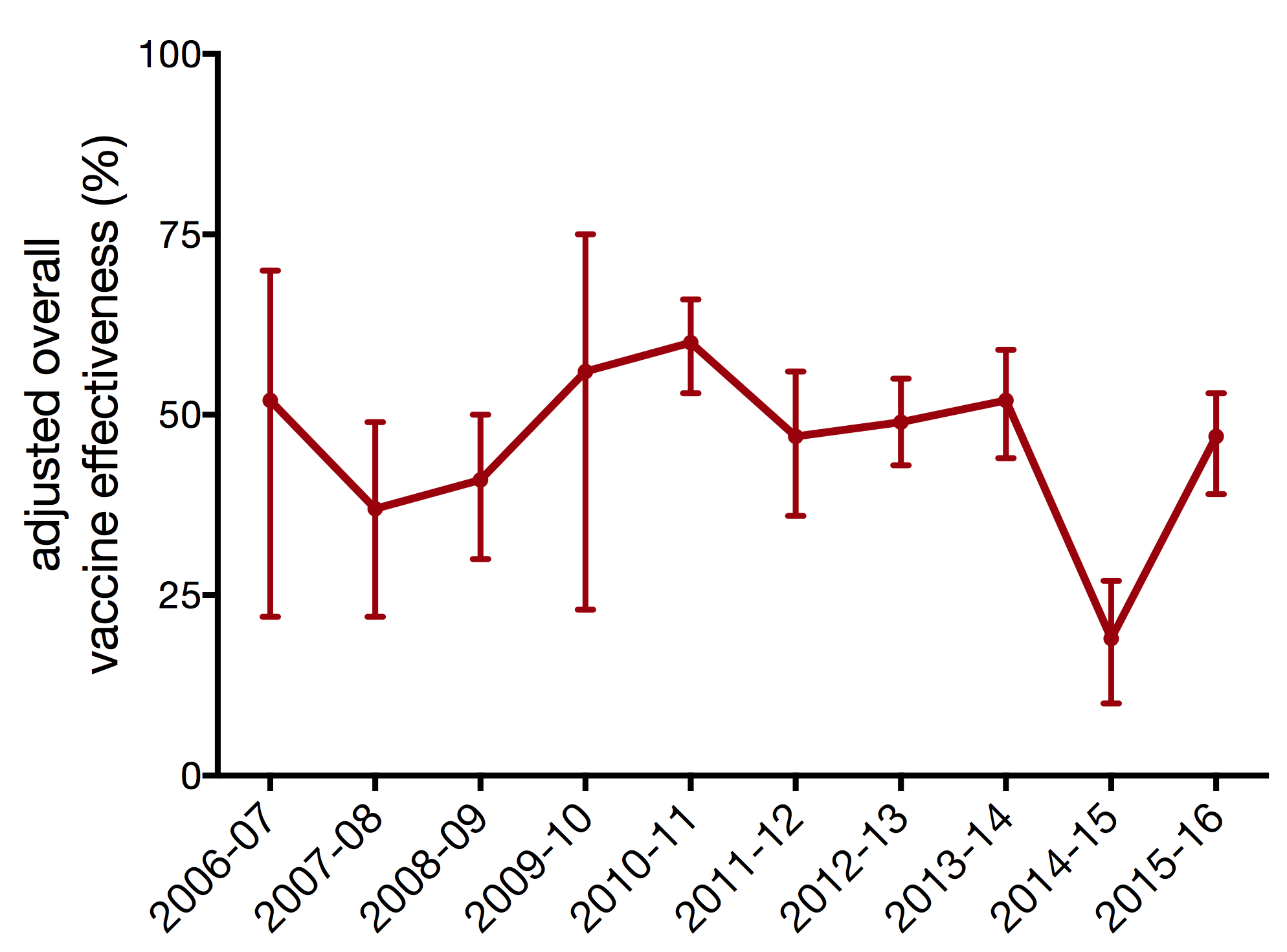
vaccine effectiveness
protecting against influenza
- sterilizing protection - prevent replication of the virus in the host
- non-sterilizing protection - protection against disease, reducing disease
- cross-reactivity - immune responses reactive against multiple strains or subtypes
- dependent on conservation
- HA stem is more conserved than the globular head
- influenza internal proteins NP and M are highly conserved
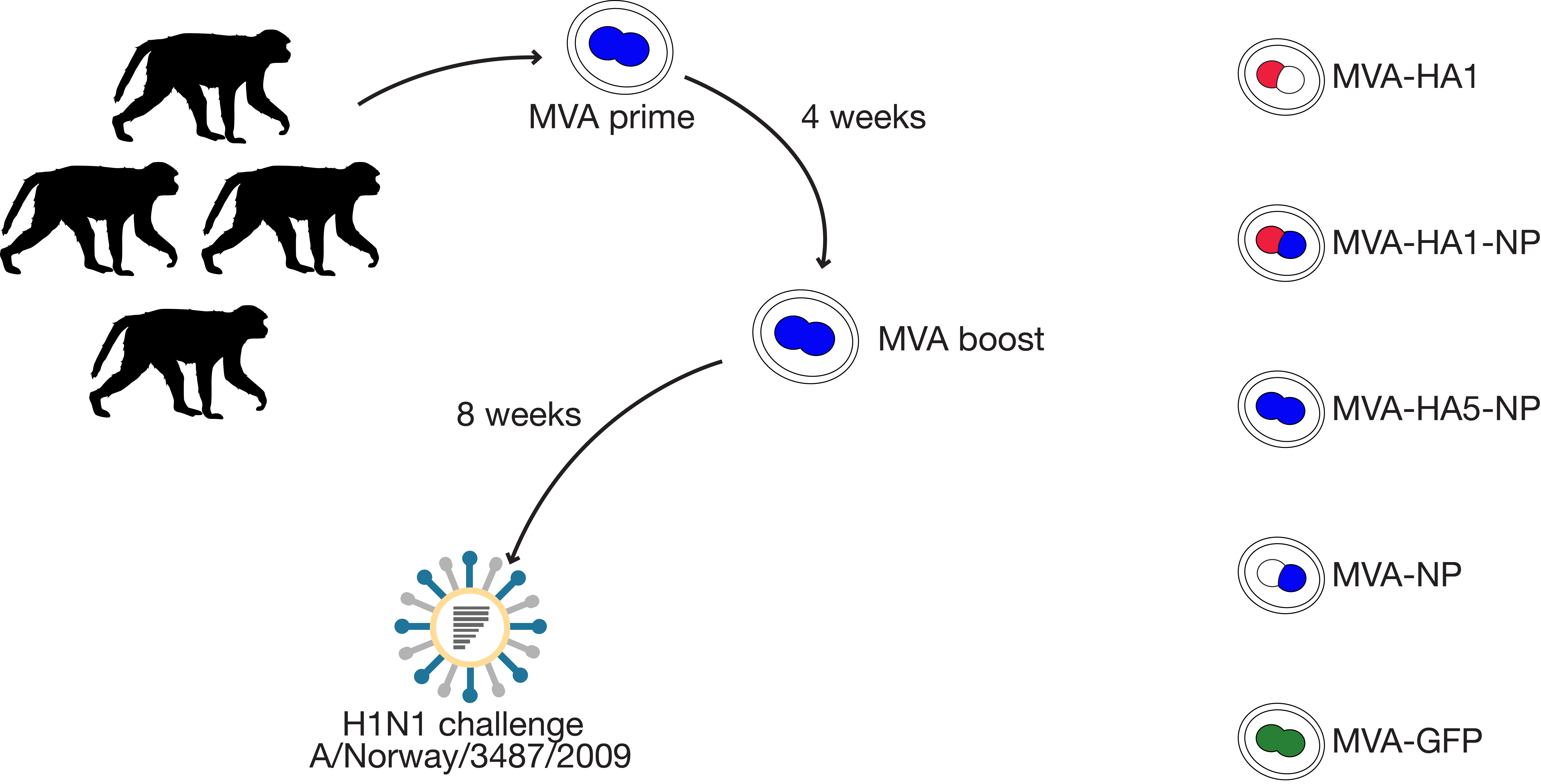
can vaccines stimulate a response that provides protection against different influenza subtypes?

modified vaccinia Ankara (MVA) vector to induce protective cytotoxic T cell responses
- attenuated poxvirus
- safe already approved for use in humans, only replicates in culture
- very immunogenic

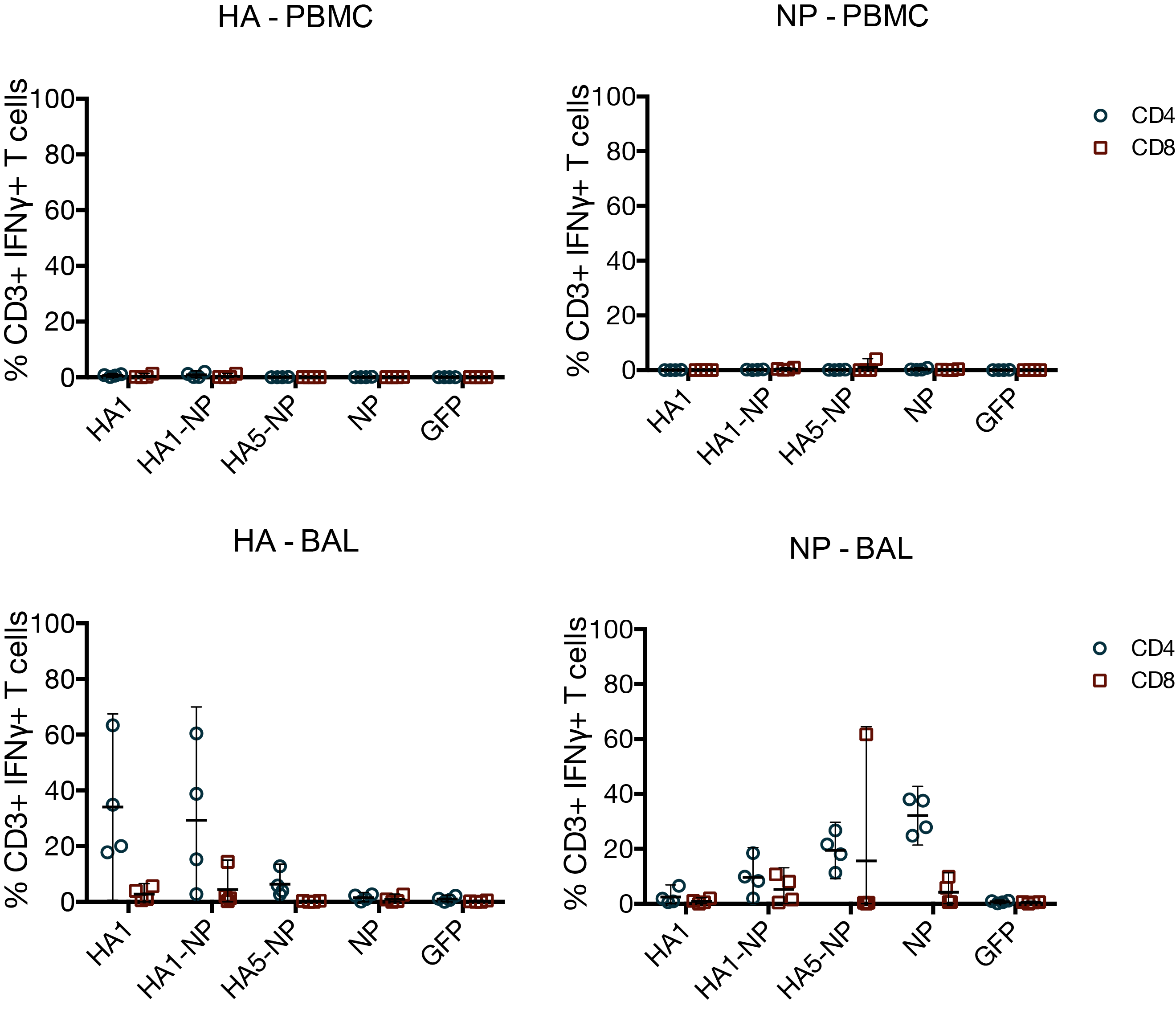
MVA vaccines have little effect on T cells
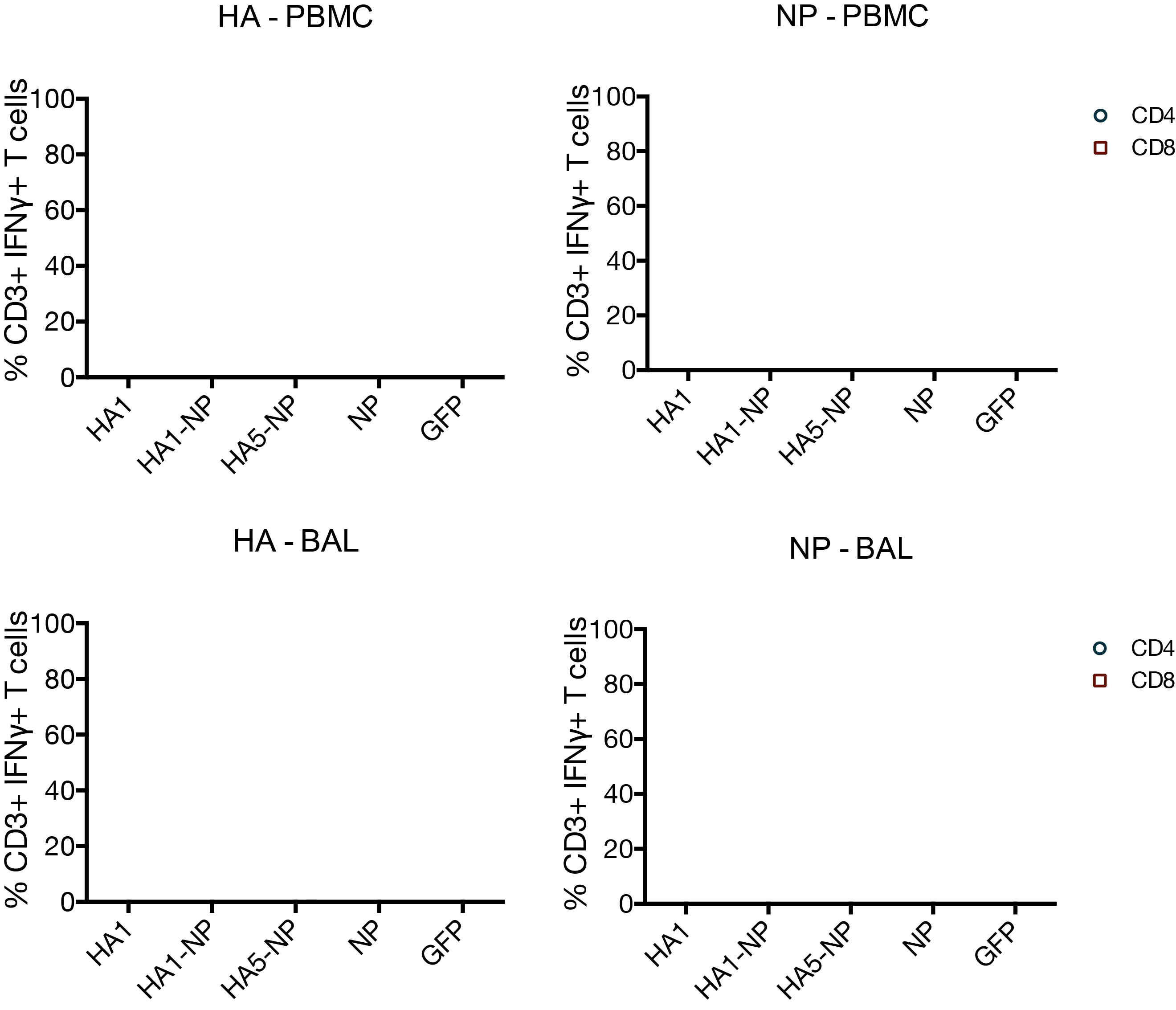
MVA vaccines have little effect on T cells
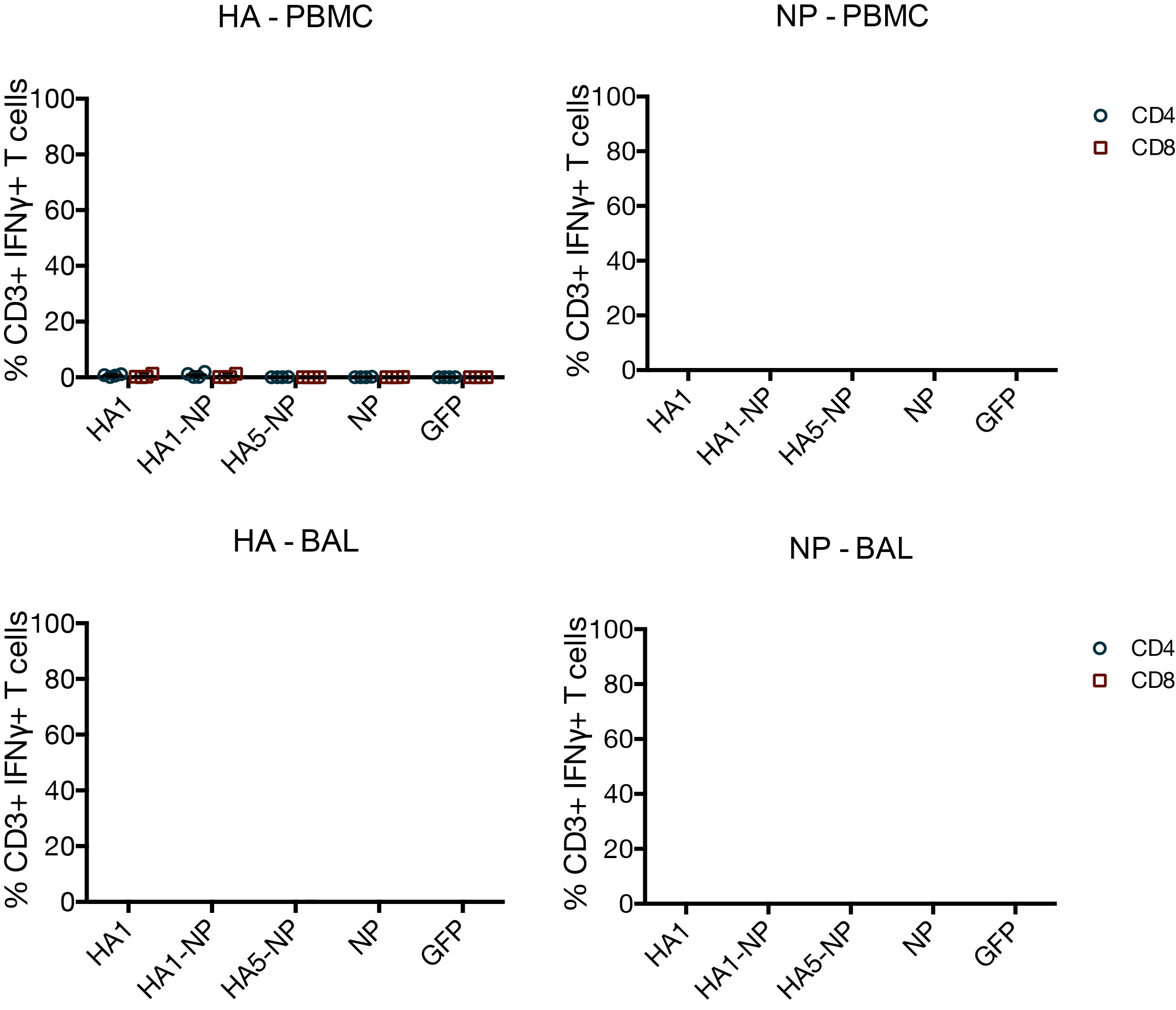
MVA vaccines have little effect on T cells
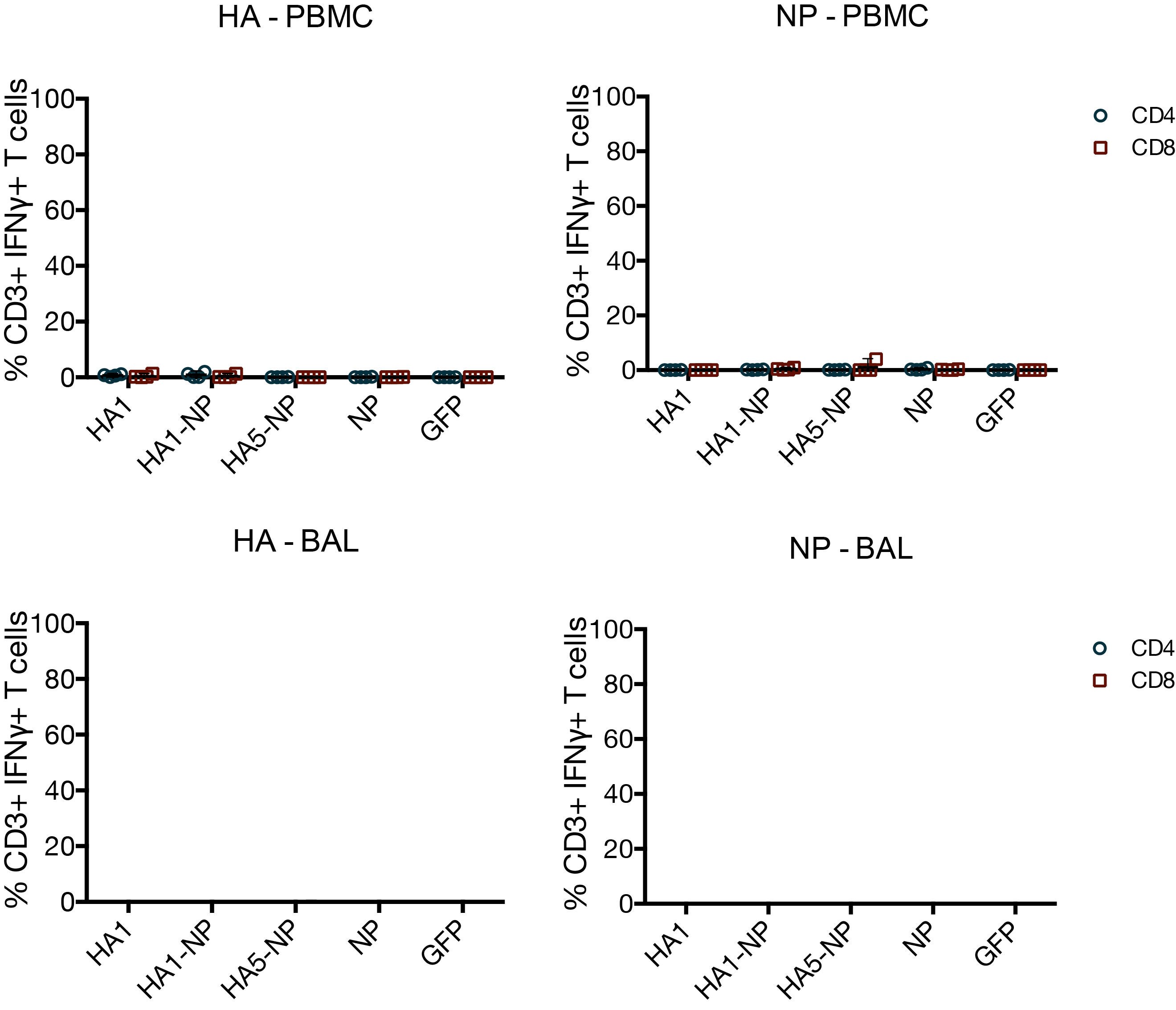
MVA vaccines have little effect on T cells
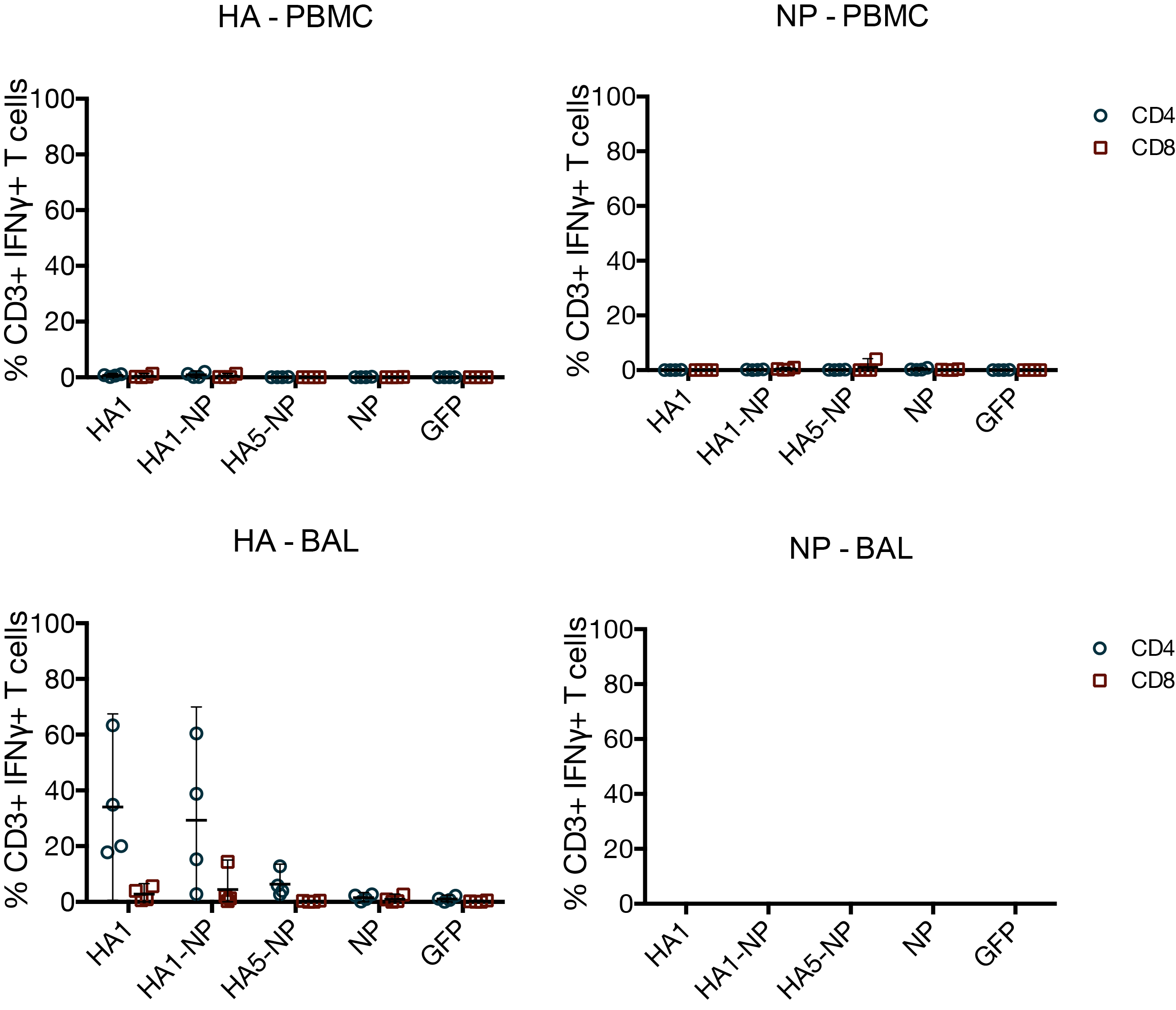
MVA vaccines have little effect on T cells
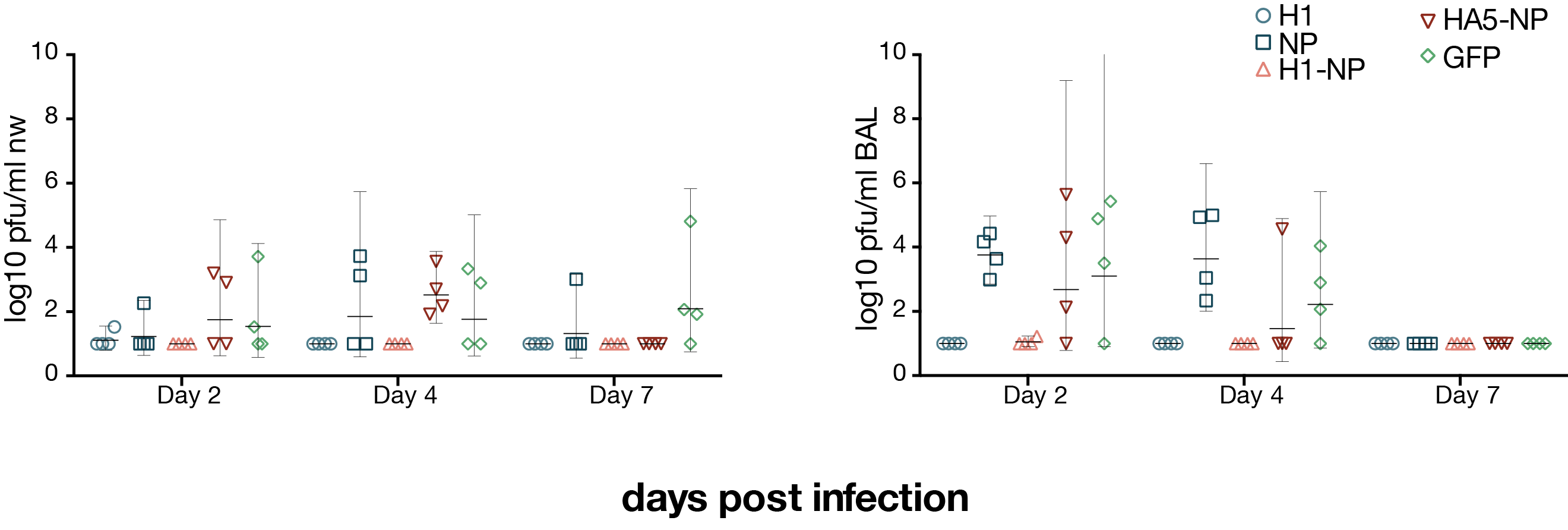
viral loads after H1N1 challenge
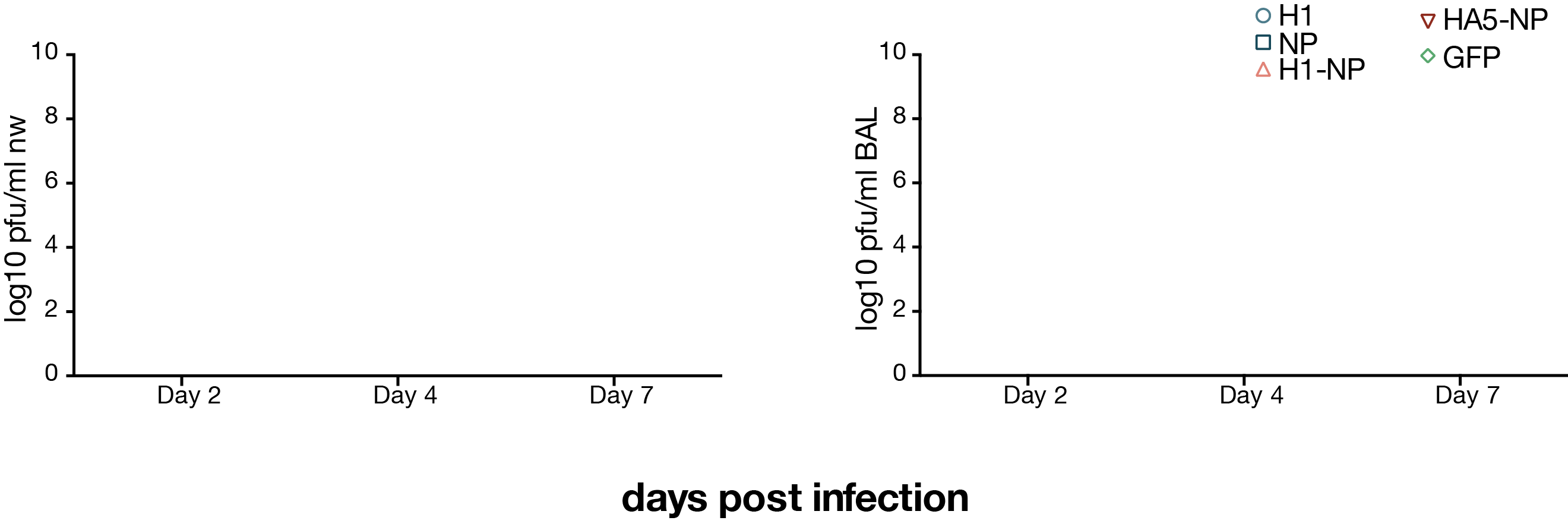
viral loads after H1N1 challenge
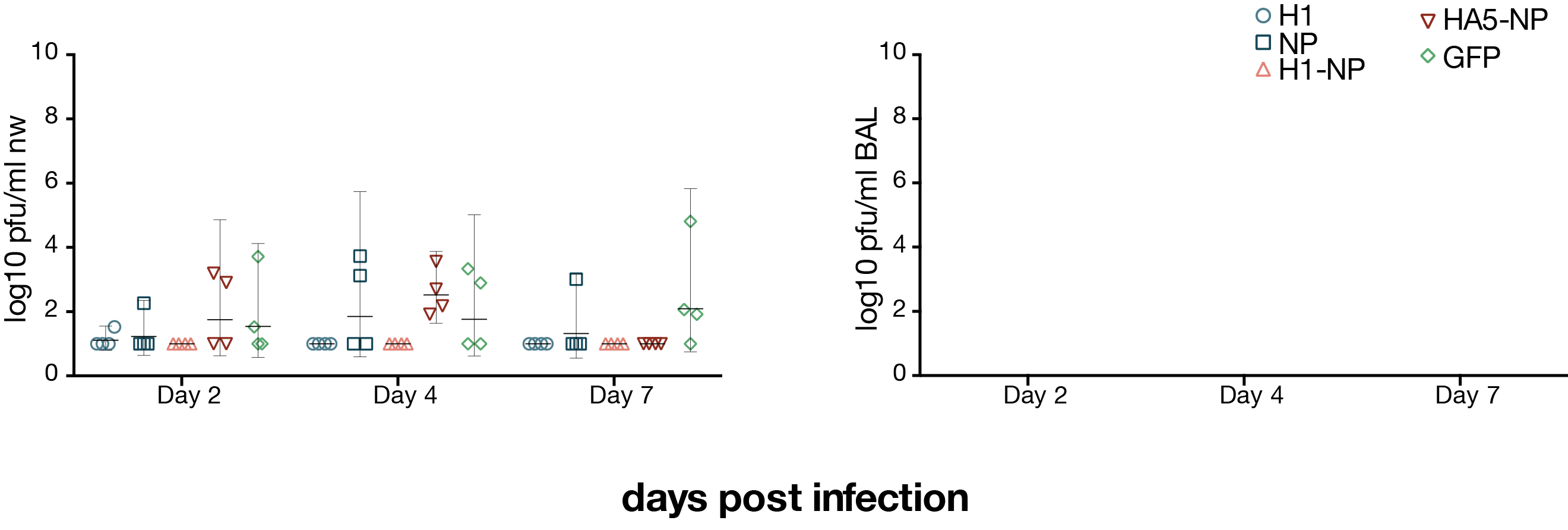
viral loads after H1N1 challenge
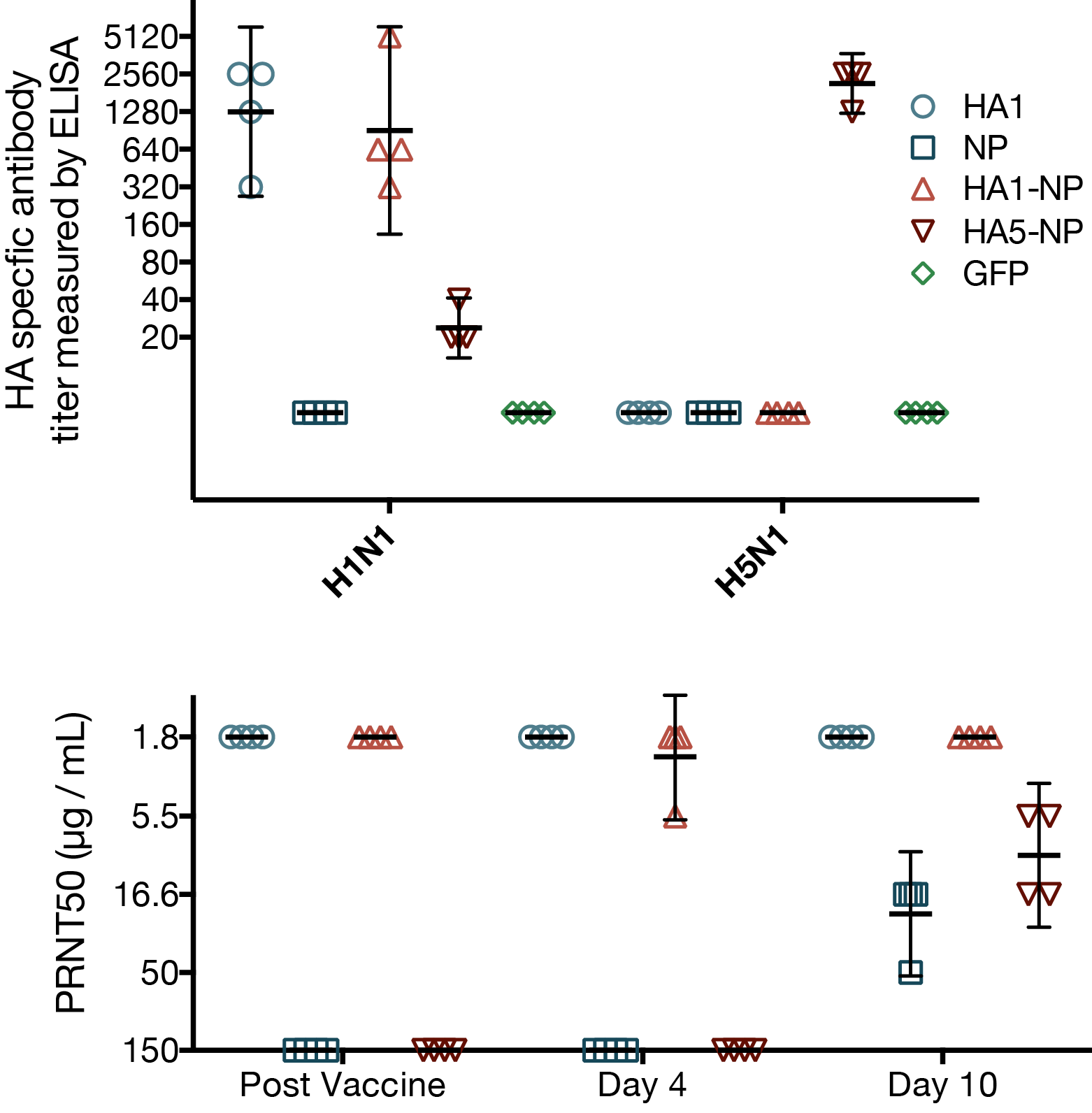
MVA vaccines unable to elicit cross-reactive neutralizing antibodies

MVA vaccines unable to elicit cross-reactive neutralizing antibodies
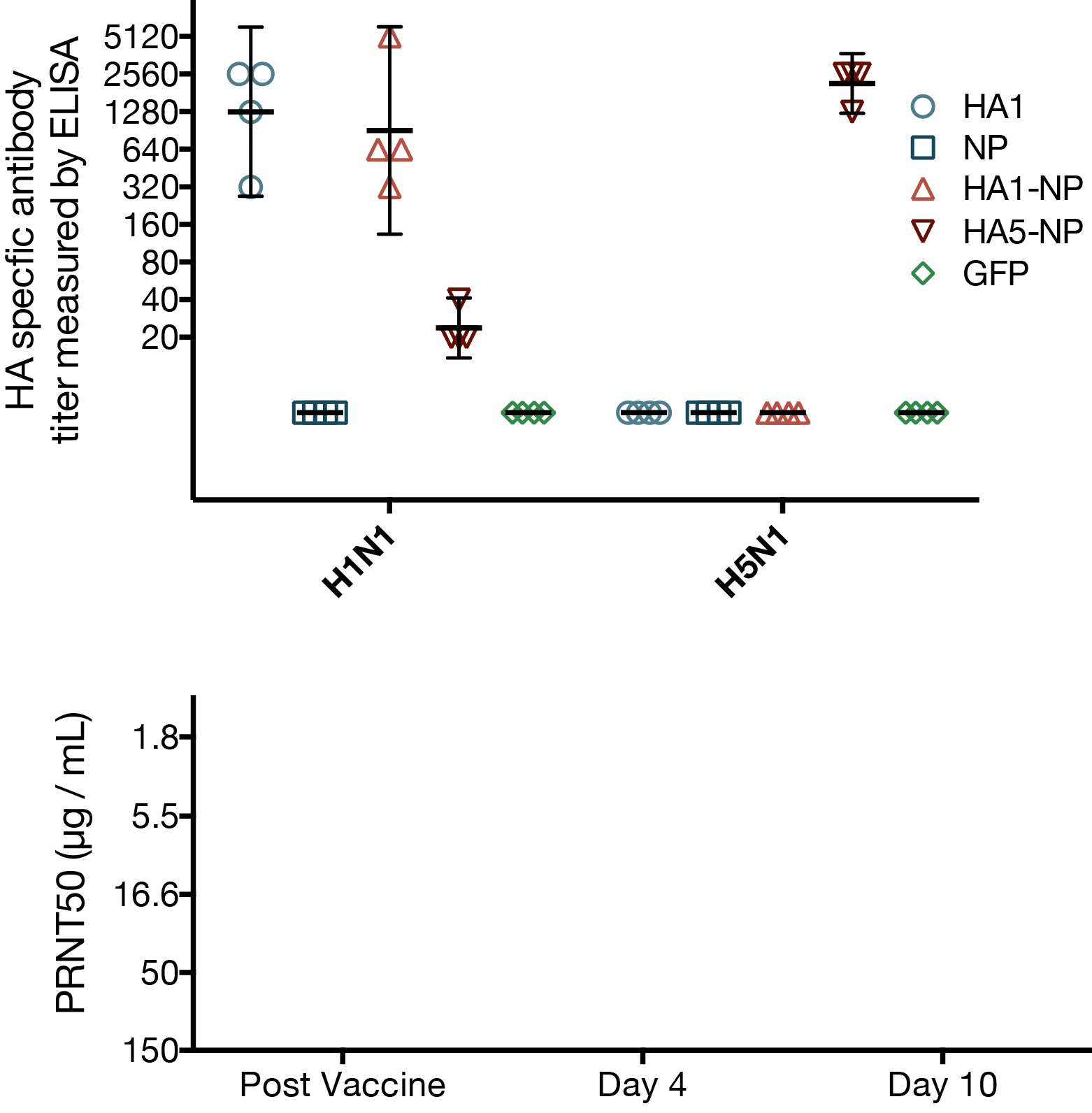
MVA vaccines unable to elicit cross-reactive neutralizing antibodies
adaptive response - antibodies

- role: recognize antigens
- function:
- neutralization - blocks functional parts of a bacteria or virus
- opsonization - tag foreign targets boosting the kinetics of phagocytosis
- compliment activation - initialization of the compliment membrane attack complex
- activate effector cells - engagement and activation of an immune effector cell
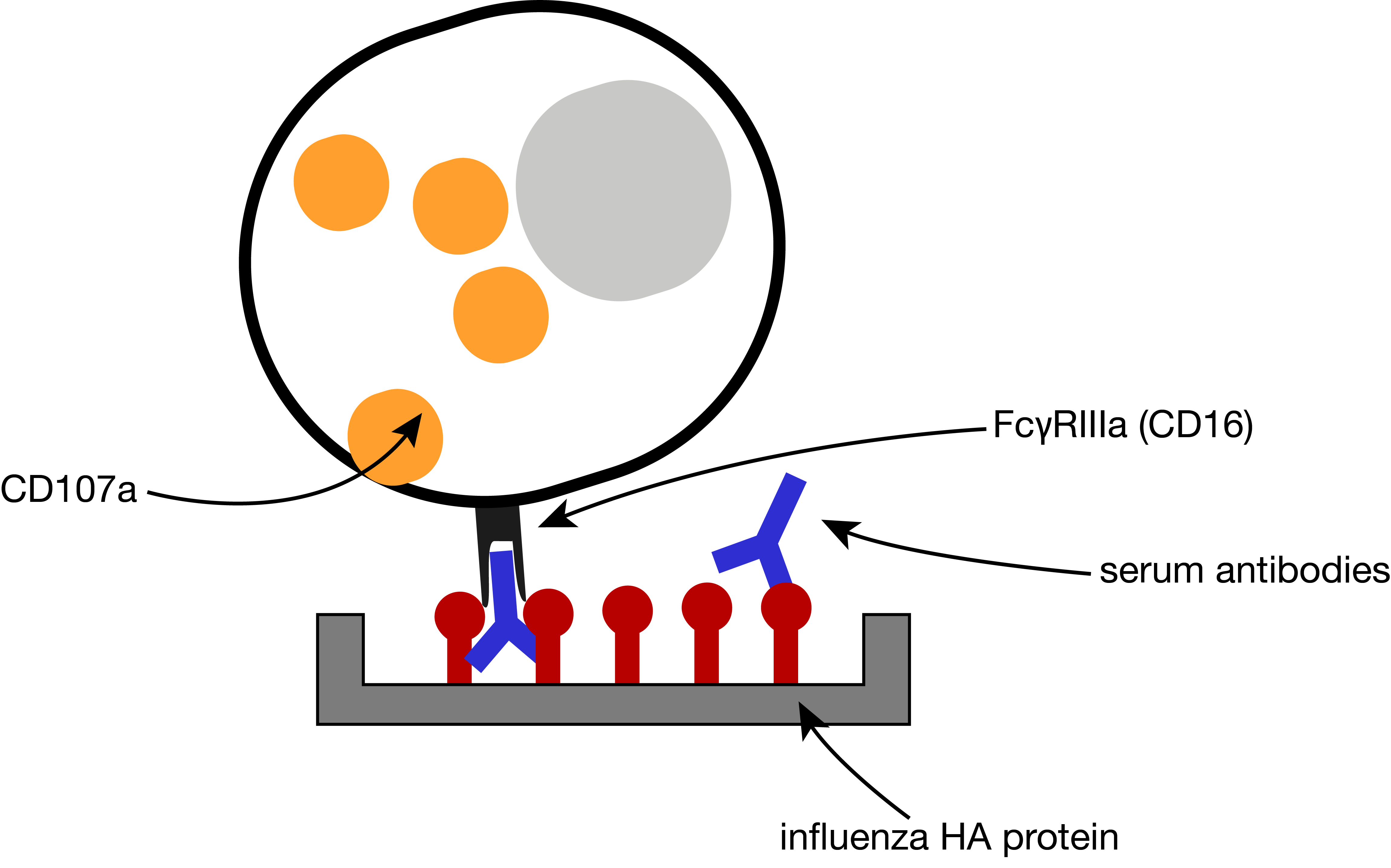
antibody-dependent cell-mediated cytotoxicity (ADCC)
antibody-dependent cell-mediated cytotoxicity (ADCC)
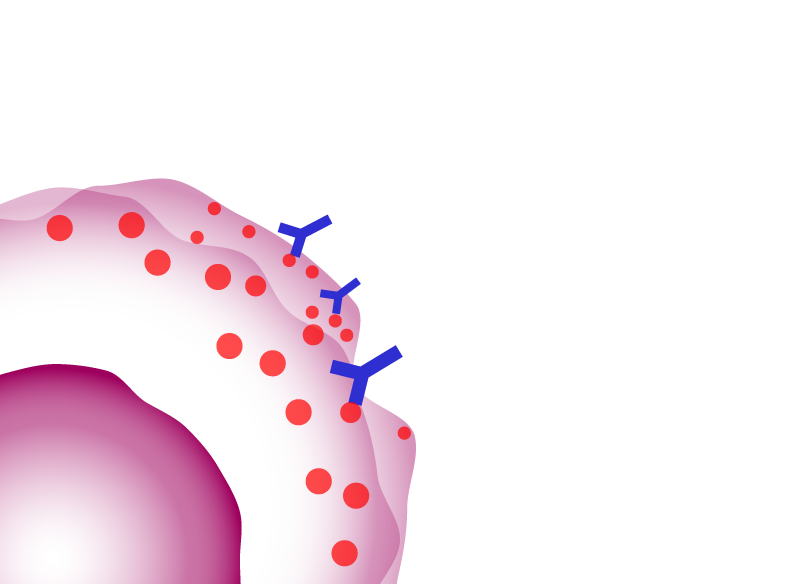
antibody-dependent cell-mediated cytotoxicity (ADCC)
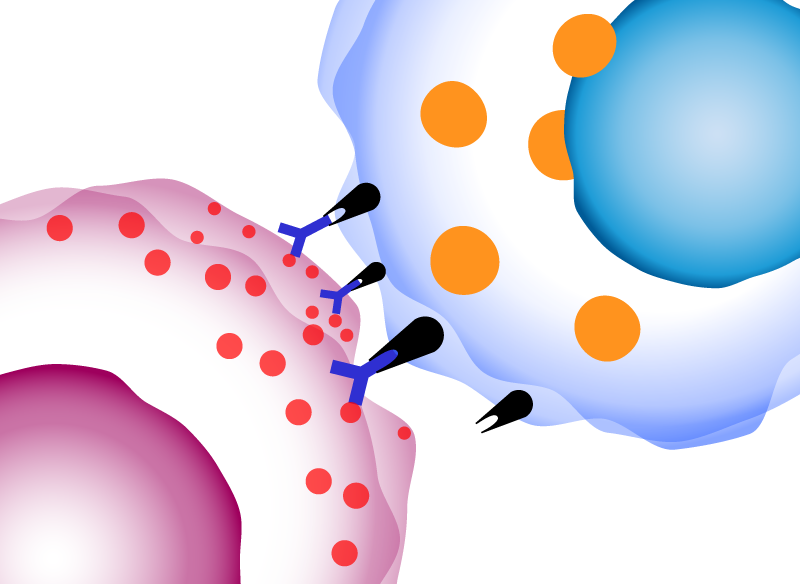
ADCC assay
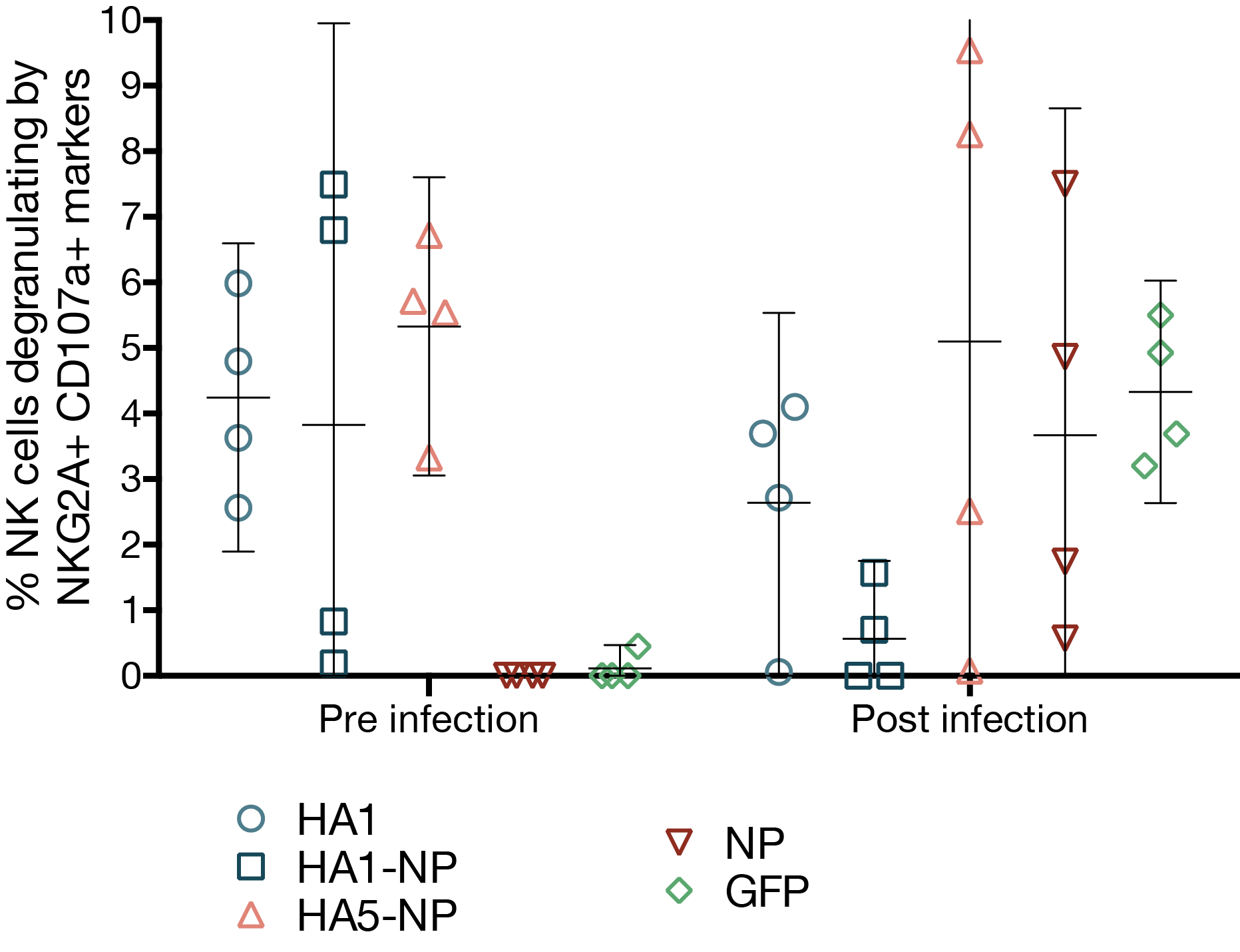
MVA vaccines elicit antibodies capable of ADCC
MVA vaccines elicit antibodies capable of ADCC
conclusions from non-human primate study
- MVA-HA5-NP induces cross-reactive antibodies against an H1N1 virus
- antibodies elicited by vaccination were capable of ADCC
- ADCC antibodies may contribute to faster clearance of influenza virus
- MVA vaccines did not induce potent T cell responses
does ADCC play a role in human responses to seasonal influenza vaccination?
vaccine effectiveness

study timeline
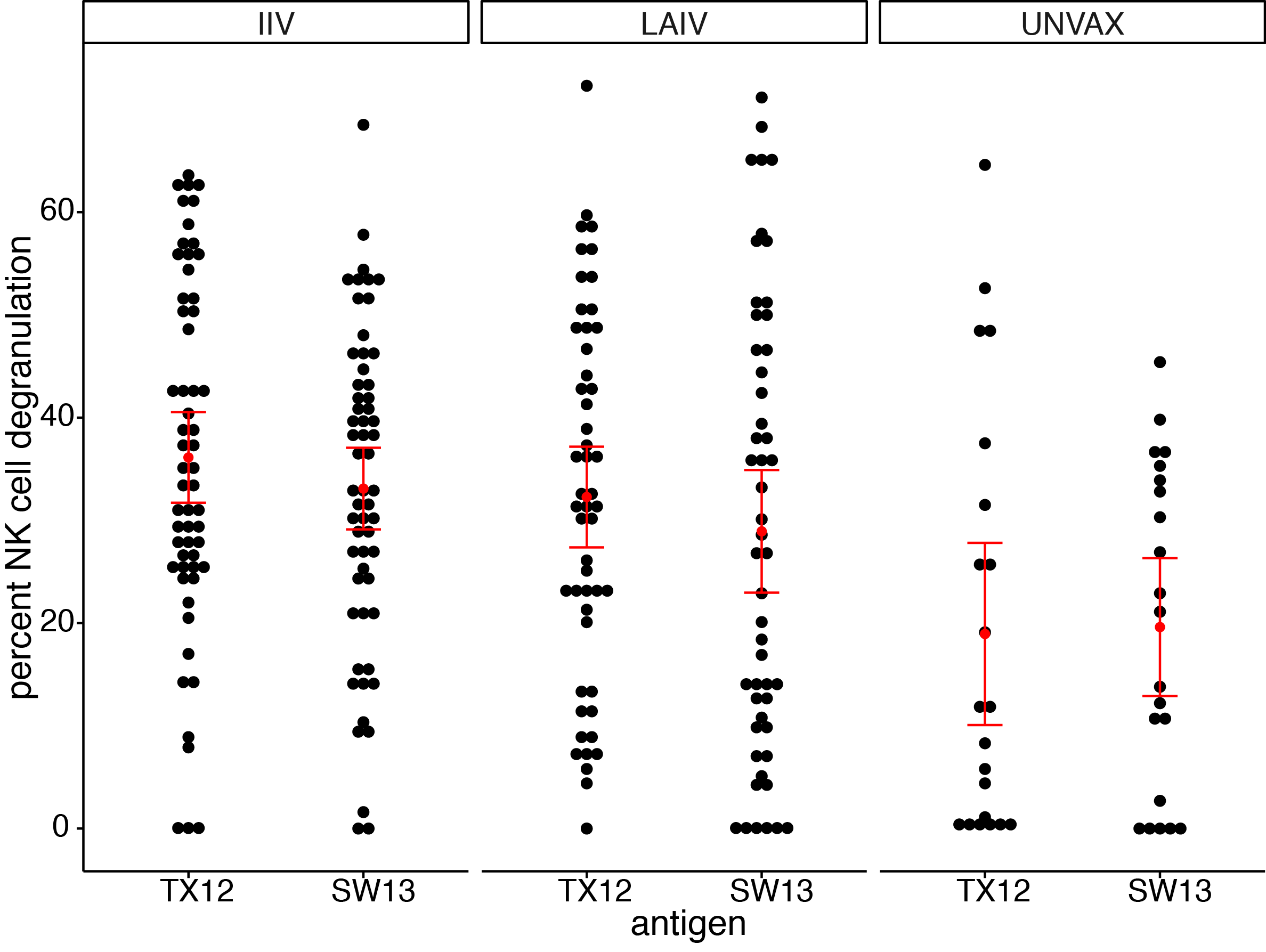
ADCC antibodies present at baseline
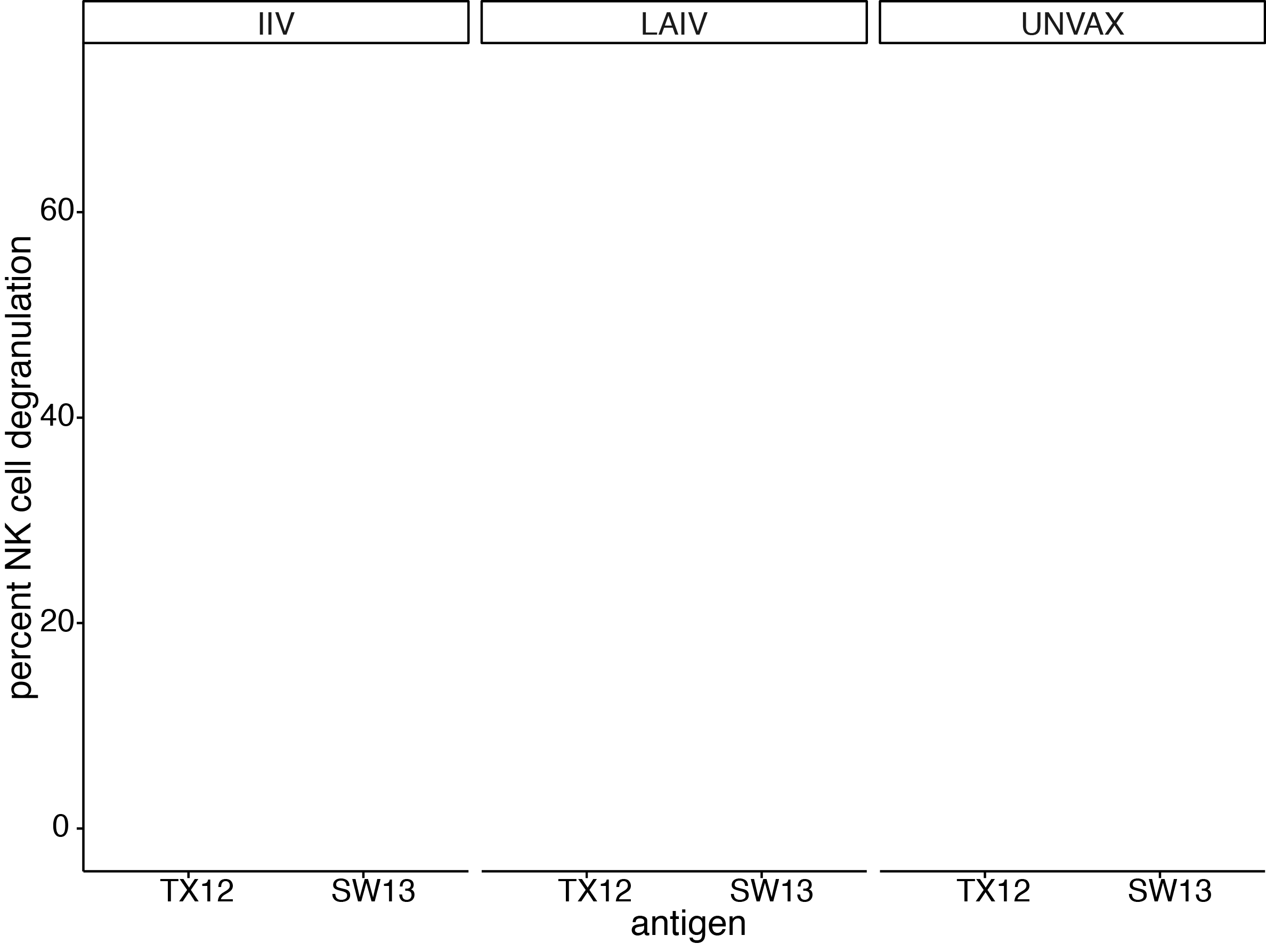
ADCC antibodies present at baseline
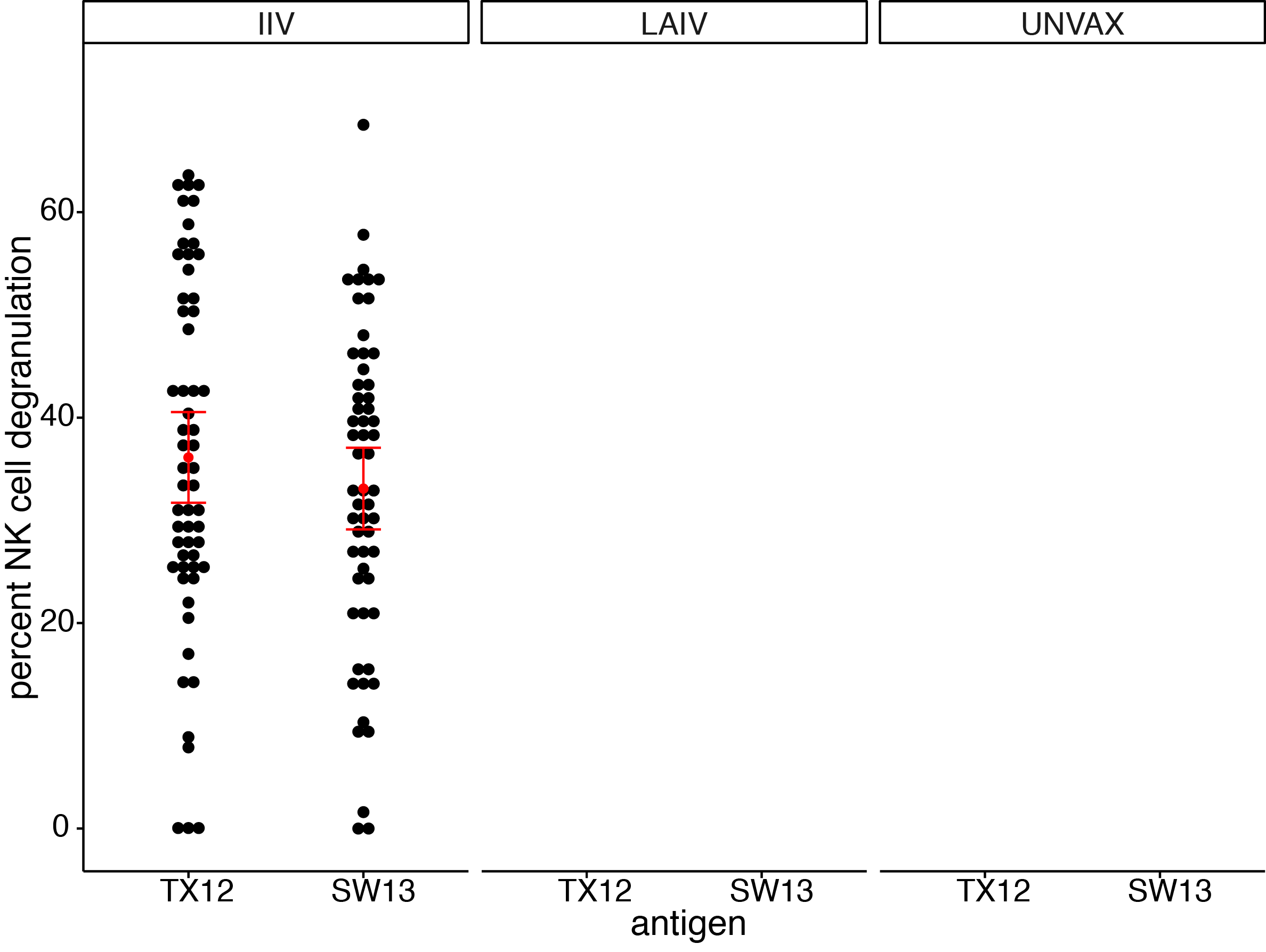
ADCC antibodies present at baseline
ADCC antibodies present at baseline
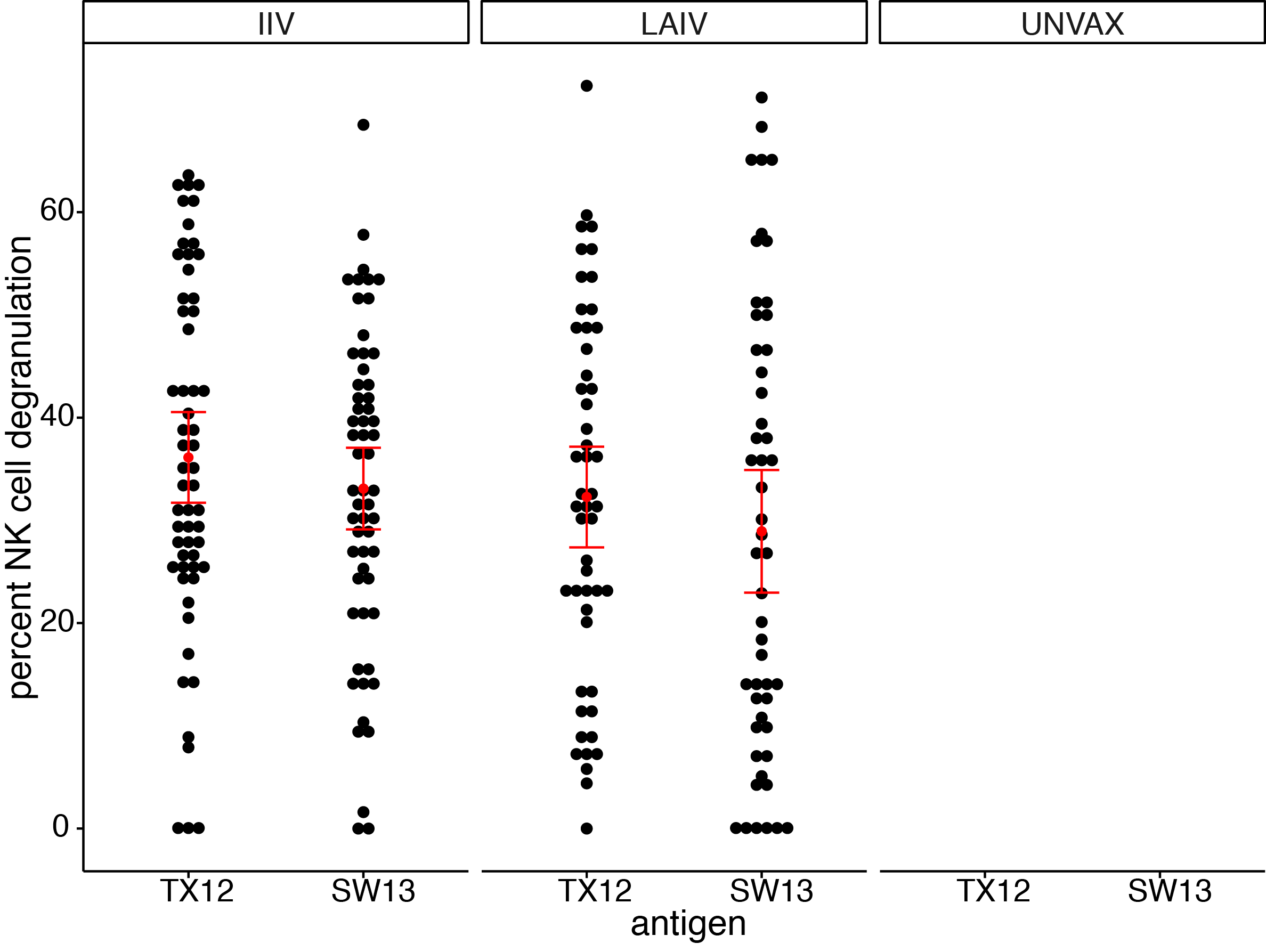
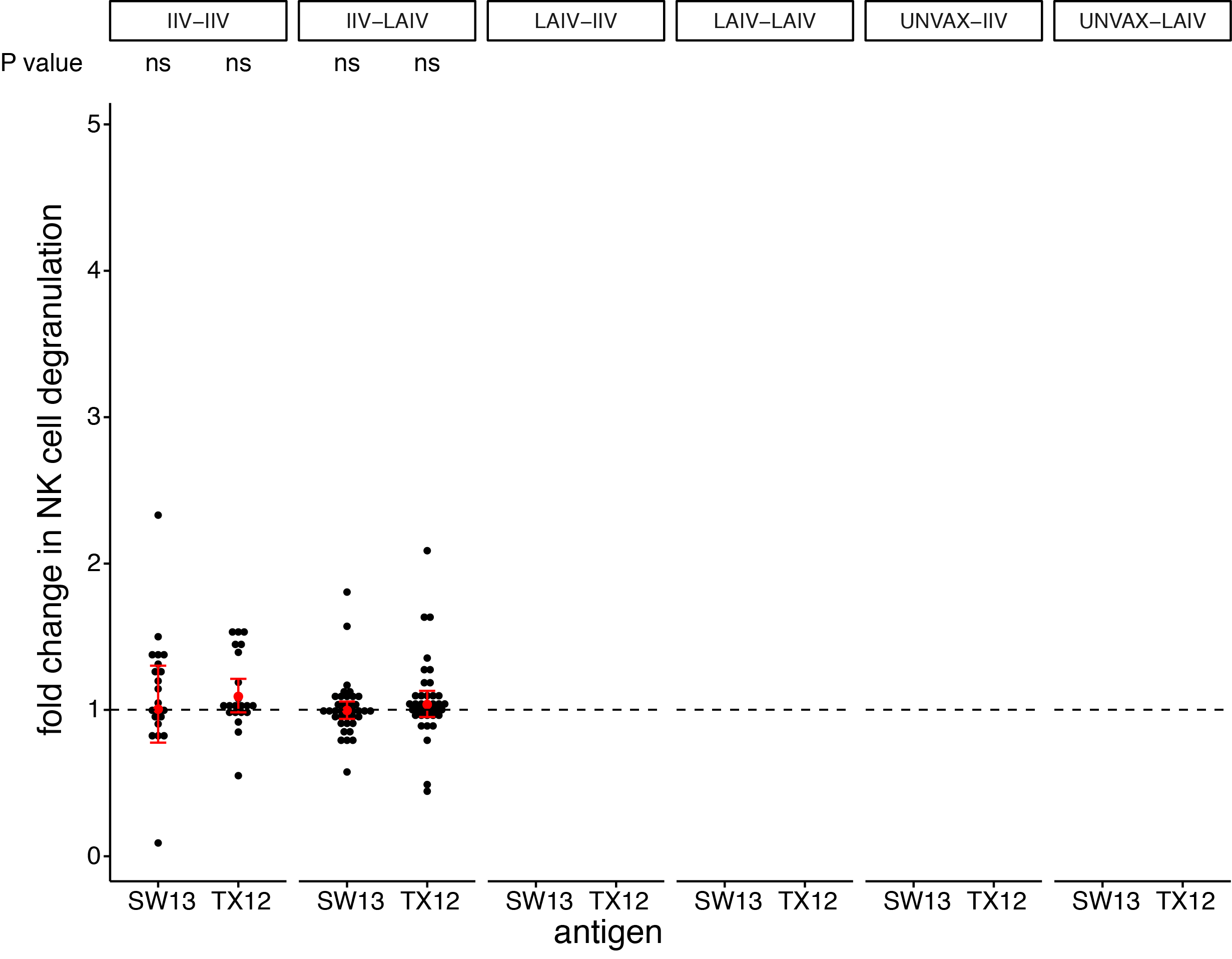
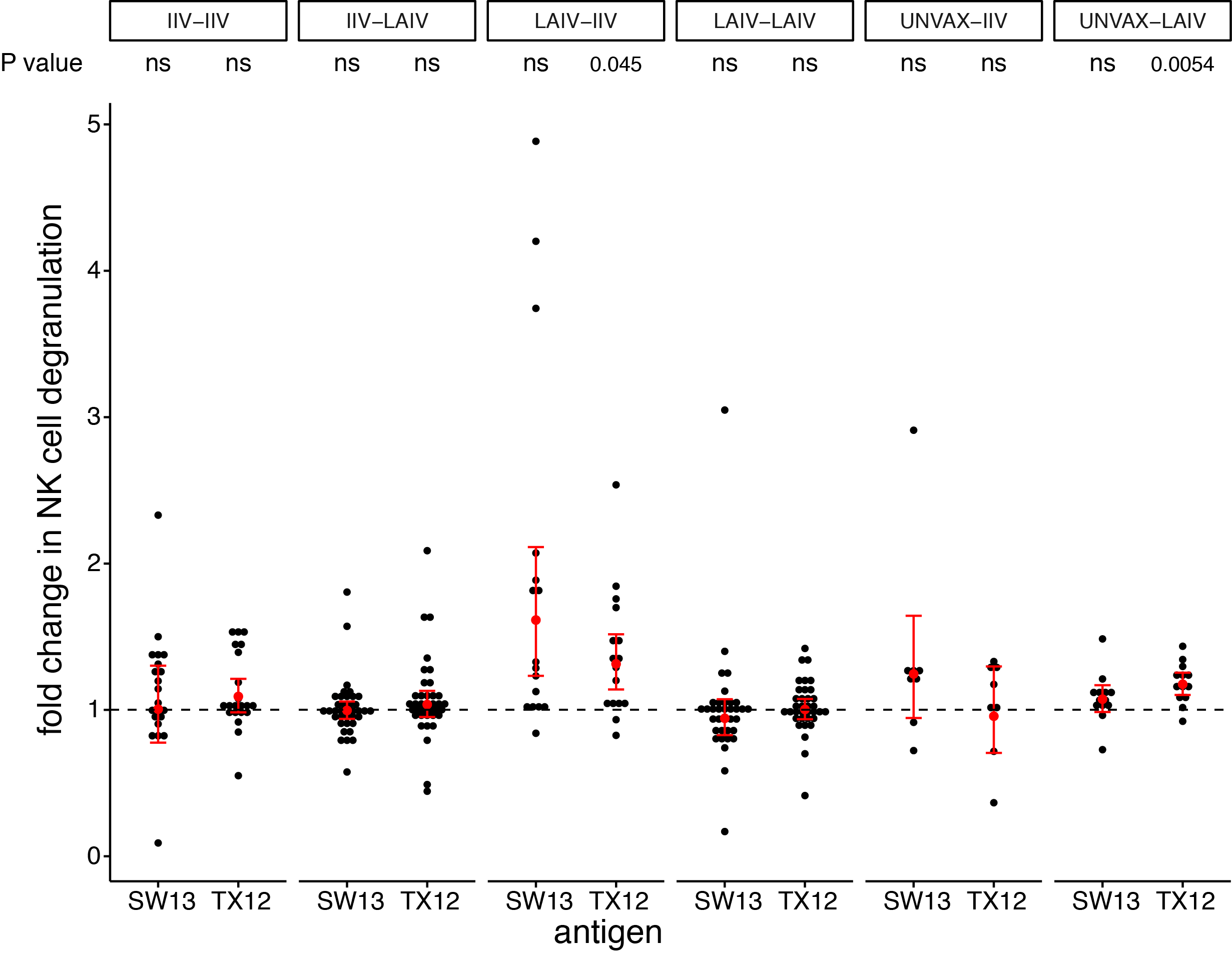
seasonal vaccines have minimal effects on ADCC antibodies

seasonal vaccines have minimal effects on ADCC antibodies

seasonal vaccines have minimal effects on ADCC antibodies
seasonal vaccines have minimal effects on ADCC antibodies
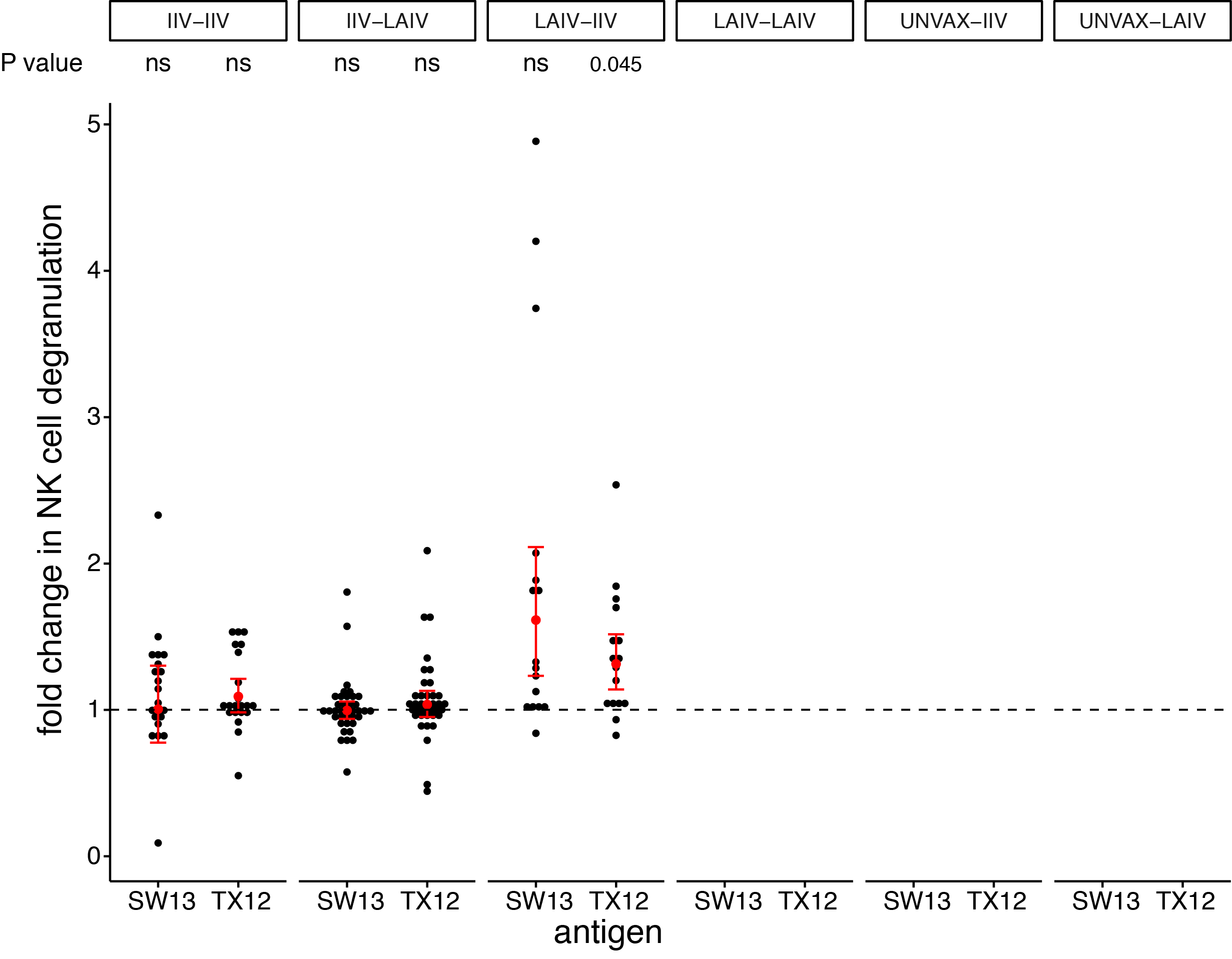
seasonal vaccines have minimal effects on ADCC antibodies
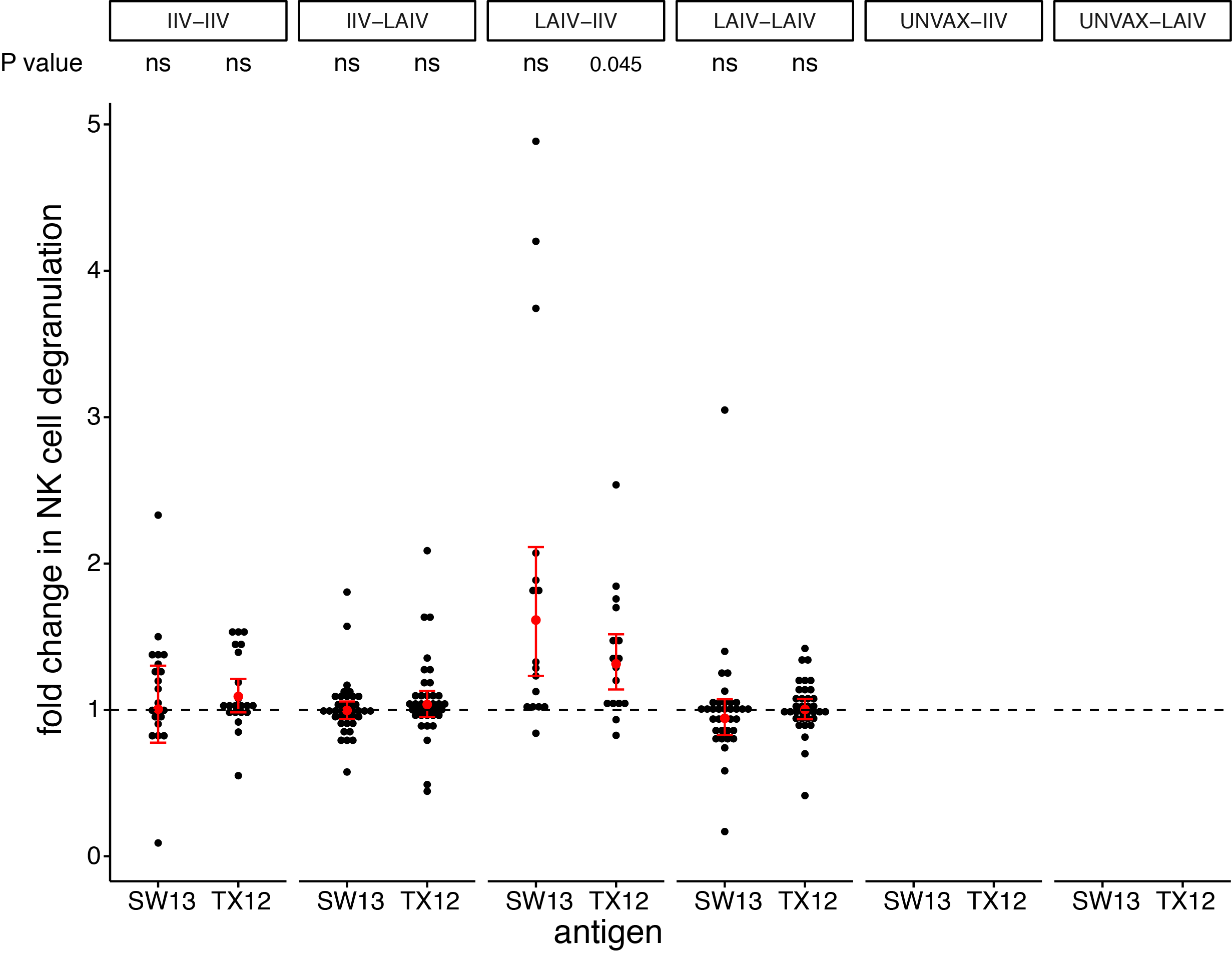
seasonal vaccines have minimal effects on ADCC antibodies
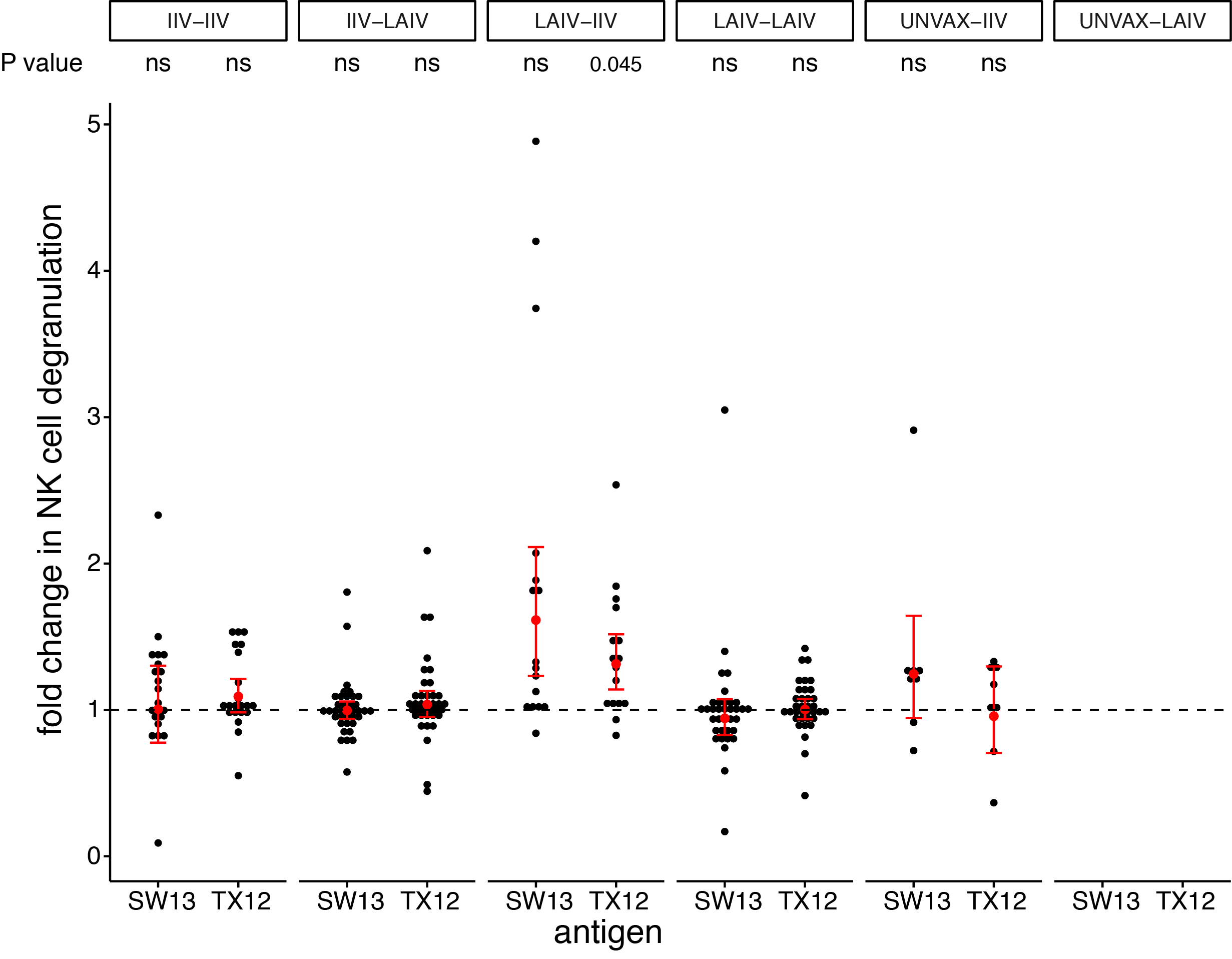
seasonal vaccines have minimal effects on ADCC antibodies
conclusions
- cross-reactive ADCC antibodies are present before vaccination
- vaccines are only capable of a modest boost to ADCC antibodies
issues faced by influenza vaccines
- viral evolution through drift and shift
- weakly immunogenic
- pre-existing immunity
pre-existing immunity in human studies
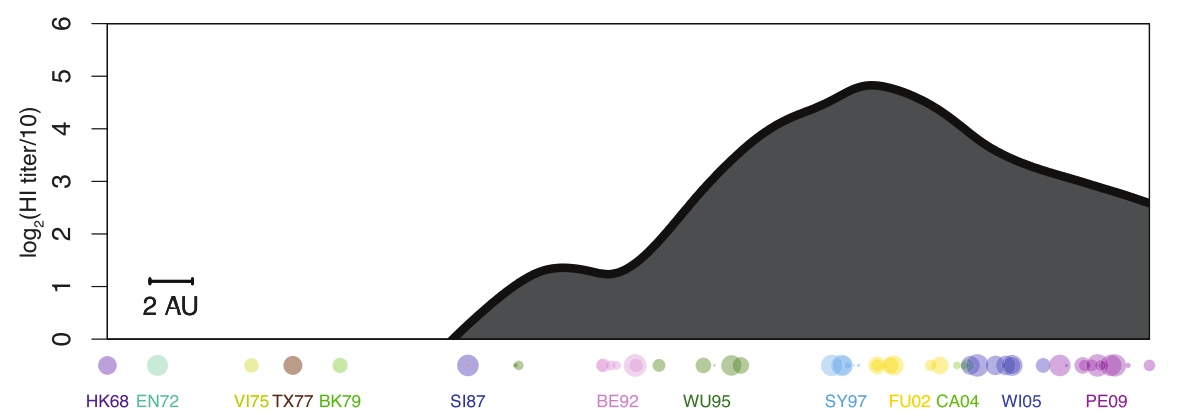
pre-existing immunity in animal studies
original antigenic sin
- mechanism
- antigen trapping
- antigen presentation
- prevention
- adjuvants to shift response from B cells to dendritic cells
- antigens delivered by vector
a better understanding of sin for a better vaccine
- new technologies to determine mechanisms
- new vaccines to stimulate the "non-natural response"
- develop a better vaccines through understanding virus host interactions
on the importance of influenza vaccines

acknowledgements
- Friedrich Lab
- Tom Friedrich
- James Mutschler
- Louise Moncla
- Andrea Weiler
- Gabrielle Lehrer-Brey
- Jorge Dinis
- Marshfield Clinic Research Foundation
- Edward Belongia
- Huong McLean
- Jennifer Meece
- Jennifer King
- Others
- Jason Weinfurter
- Sinthujan Jegaskanda
- Stephen Kent
- Jorge Osorio
- Tom Friedrich
- James Mutschler
- Louise Moncla
- Andrea Weiler
- Gabrielle Lehrer-Brey
- Jorge Dinis
- Edward Belongia
- Huong McLean
- Jennifer Meece
- Jennifer King
- Jason Weinfurter
- Sinthujan Jegaskanda
- Stephen Kent
- Jorge Osorio
- Thesis committee
- Laura Knoll
- John Mansfield
- Nathan Sherer
- David Evans
- Laura Knoll
- John Mansfield
- Nathan Sherer
- David Evans
funding provided by:
- SciMed Graduate Research Scholars
- Molecular Biosciences Training Grant






